Abstract
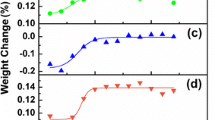
A natural graphite recommended for use in nuclear applications was analyzed using thermogravimetric analysis. The oxidation behaviour was unlike that expected for flake-like particles. The dynamic data displayed an apparent bimodal reaction rate curve as a function of temperature and degree of conversion. Nevertheless, it was possible to model this behaviour with a single rate constant, i.e. without the need for a parallel reaction type of kinetic mechanism. The approach used in this paper to model the gas–solid reaction of graphite and oxygen, provides a consistent framework to test the validity of complementary isothermal and non-isothermal data for a specific solid state reaction.














Similar content being viewed by others
Abbreviations
- E A :
-
Activation energy (J/mol)
- k o :
-
Arrhenius pre-exponential (1/s)
- R :
-
Gas constant (J/mol K)
- \( {\rm P}_{{O_{2} }} \) :
-
Partial pressure of oxygen (kPa)
- t:
-
Time (s)
- T:
-
Temperature (K)
- Y :
-
Mole fraction (−)
- α:
-
Dimensionless conversion (–)
- β:
-
Temperature scan rate (K/s)
- 0:
-
Initial
- m, n:
-
Indices
- r :
-
Reaction order in O2
References
Galwey AK. What can we learn about the mechanisms of thermal decompositions of solids from kinetic measurements?. J Therm Anal Calorim. 2008;92:967–83.
Galwey AK. Perennial problems and promising prospects in the kinetic analysis of nonisothermal rate data. Thermochim Acta. 2003;407:93–103.
Galwey AK. Thermochim Acta. Is the science of thermal analysis kinetics based on solid foundations? A literature appraisal. 2004;413:139–83.
Šesták J, Berggren G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim Acta. 1971;3:1–12.
Koga N. Physico-Geometric kinetics of solid-state reactions by thermal analyses. J Therm Anal. 1997;49:45–56.
Anderson HL, Kemmler A, Höhne GWH, Heldt K, Strey R. Round robin test on the kinetic evaluation of a complex solid state reaction from 13 European laboratories. Part 1. Kinetic TG-analysis. Thermochim Acta. 1999;332:33–53.
Koga N, Tanaka H. A physico-geometric approach to the kinetics of solid-state reactions as exemplified by the thermal dehydration and decomposition of inorganic solids. Thermochim Acta. 2002;388:41–61.
Vyazovkin S. Kinetic concepts of thermally simulated reactions in solids: a view from a historical perspective. Int Rev Phys Chem. 2000;19:45–60.
Galwey AK, Brown ME. Application of the Arrhenius equation to solid state kinetics: can this be justified?. Thermochim Acta. 2002;386:91–8.
Gimzewski E. The relationship between oxidation induction temperatures and times for petroleum products. Thermochim Acta. 1992;198:133–40.
Zaghib K, Song X, Kinoshita K. Thermal analysis of the oxidation of natural graphite: isothermal kinetic studies. Thermochim Acta. 2001;371:57–64.
Galwey AK. What theoretical and/or chemical significance is to be attached to the magnitude of an activation energy determined for a solid-state composition? J Therm Anal Calorim. 2006;86:267–86.
Galwey AK. Eradicating erroneous Arrhenius arithmetic. Thermochim Acta. 2003;399:1–29.
Hurt RH, Haynes BS. On the origin of power-law kinetics in carbon oxidation. Proc Combust Inst. 2005;30:2161–8.
Moormann R, Hinssen H-K. Basic studies in the field of high-temperature engineering. Presented at second information exchange meeting, Paris, France, 2001, p. 243–54.
Hennig GR. Catalytic oxidation of graphite. J Inorg Nucl Chem. 1962;24:1129–37.
McKee DW. The catalyzed gasification reactions of carbon. Chem Phys Carbon. 1981;16:183–99.
McKee DW, Chatterji D. The catalytic behaviour of alkali metal carbonates and oxides in graphite oxidation reactions. Carbon. 1975;13:381–90.
Ranish JM, Walker PL Jr. Models for roughening of graphite during its catalyzed gasification. Carbon. 1990;28:887–96.
Acknowledgements
This work is based upon research supported by PBMR and the South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation. Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors and therefore the PBMR, NRF ad DST do not accept any liability with regard thereto.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix 1
Rights and permissions
About this article
Cite this article
Badenhorst, H., Rand, B. & Focke, W.W. Modelling of natural graphite oxidation using thermal analysis techniques. J Therm Anal Calorim 99, 211–228 (2010). https://doi.org/10.1007/s10973-009-0095-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0095-3