Abstract
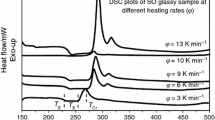
The present paper reports on the effect of MoO3 on the glass transition, thermal stability and crystallization kinetics for (40PbO–20Sb2O3–40As2O3)100−x –(MoO3) x (x = 0, 0.25, 0.5, 0.75 and 1 mol%) glasses. Differential scanning calorimetry (DSC) results under non-isothermal conditions for the studied glasses were reported and discussed. The values of the glass transition temperature (T g) and the peak temperature of crystallization (T p) are found to be dependent on heating rate and MoO3 content. From the compositional dependence and the heating rate dependence of T g and T p, the values of the activation energy for glass transition (E g) and the activation energy for crystallization (E c) were evaluated and discussed. Thermal stability for (40PbO–20Sb2O3–40As2O3)100−x –(MoO3) x glasses has been evaluated using various thermal stability criteria such as ΔT, H r , H g and S. Moreover, in the present work, the K r(T) criterion has been considered for the evaluation of glass stability from DSC data. The stability criteria increases with increasing MoO3 content up to x = 0.5 mol%, and decreases beyond this limit.




Similar content being viewed by others
References
Nalin M, Poulain M, Ribeiro JL, Messaddeq Y. Antimony oxide based glasses. J Non-Cryst Solids. 2001;284:110–6.
Poirier G, Poulain M, Poulain M. Copper and lead halogeno-antimoniate glasses. J Non-Cryst Solids. 2001;284:117–22.
Fargin E, Berthereau A, Cardinal T, Le Flem G, Ducasse L, Canioni L, et al. Optical non-linearity in oxide glasses. J Non-Cryst Solids. 1996;203:96–101.
Little Flower G, Sahaya Baskaran G, Krishna Mohan N, Veeraiah N. The structural role of tungsten ions in PbO–Sb22O3–As2O3 glass-system by means of spectroscopic investigations. Mater Chem Phys. 2006;100:211–6.
Terashima K, Hashimoto T, Uchino T, Yoko T. Structure and nonlinear optical properties of Sb2O3-B2O3 binary glasses. J Ceram Soc Jpn. 1996;104:1008–14.
Wells A. Structural inorganic chemistry. 4th ed. Oxford: Clarendon Press; 1975. p. 88.
Cotton FA, Wilkinson G, Murillo CA, Bochmann M. Advanced inorganic chemistry. New York: Wiley; 1999. p. 493.
Sabadel JC, Armand P, Cachau-Herreillat D, Baldeck P, Doclot O, Ibanez A, et al. Structural and nonlinear optical characterizations of tellurium oxide-based glasses: TeO2–BaO–TiO2. J Solid State Chem. 1997;132:411–9.
Iordanova R, Dimitrov V, Klissurski D. Glass formation and structure of glasses in the V2O5–MoO3–Bi2O3 system. J Non-Cryst Solids. 1994;180:58–65.
Klissurski D, Pesheva Y, Abadjeva N. Multicomponent oxide catalysts for the oxidation of methanol to formaldehyde. Appl Catal. 1991;77:55–66.
Syam Prasad P, Raghavaiah BV, Balaji Rao R, Laxmikanth C, Veeraiah N. Dielectric dispersion in the PbO–MoO3–B2O3 glass system. Solid State Commun. 2004;132:235–40.
Pal M, Hirota K, Sakata H. The dc electrical conductivity of semiconducting TeO2-V2O5-MoO3. J Phys D Appl Phys. 2001;34:459–64.
El-Hofy M, Hager IZ. Ionic conductivity in MoO3-BaF2-AgI-LiF glasses. Phys Status Solidi A. 2000;182:697–707.
Shah KV, Goswami M, Aswal DK, Shrikhande VK, Gupta SK, Kothiyal GP. Effect of Na2O/K2O substitution on thermophysical properties of PbO based phosphate glasses. J Therm Anal Calorim. 2007;89:153–7.
Dahshan A, Aly KA, Dessouky MT. Thermal stability and activation energy of some compositions of Ge–Te–Cu chalcogenide system. Philos Mag. 2008;88:2399–410.
Hruby A. Evaluation of glass-forming tendency by means of DTA. Czechoslov J Phys B. 1972;22:1187–93.
Marotta A, Buri A, Branda F. Structure and devitrification behaviour of sodium, lithium and barium borophosphate glasses. J Non-Cryst Solids. 1987;95:593–9.
Zhao X, Sakka S. Glass formation and crystallization in alkali-containing fluoride glasses. J Non-Cryst Solids. 1987;95:487–94.
Borisova ZU. Glassy semiconductors. New York: Plenum; 1981. p. 231.
Sestak J. Heat as manufacturing power or the source of disorder? J Therm Anal. 2002;69:113–24.
Saad M, Poulain M. Glass forming ability criterion. Mater Sci Forum. 1987;19:11–8.
Johnson WA, Mehl KF. Reaction kinetics in processes of nucleation and growth. Trans Am Inst Mining Metall Eng. 1932;135:416–42.
Mahadevan S, Giridhar A, Singh AK. Calorimetric measurements on as-sb-se glasses. J Non-Cryst Solids. 1986;88:11–34.
Matusita K, Komatsu T, Yokota R. Kinetics of non-isothermal crystallization process and activation energy for crystal growth in amorphous materials. J Mater Sci. 1984;19:291–6.
Surinach S, Baro MD, Clavaguera-Mora MT, Clavaguera N. Glass formation and crystallization in the GeSe2-Sb2Te3 system. J Mater Sci. 1984;19:3005–12.
Young D, Jiang Z. Relationship between regions of glass formation and pseudoeutectic regions. Phy Chem Glasses. 1990;31:161–5.
ICDD View 2006 Release. Cards No. 00-018-0755, 01-074-0054, 01-077-0295, 00-022-0421, 00-024-0668.
Lide D. CRC handbook of chemistry and physics. 84th ed. Boca Raton: CRC Press; 2004. p. 9–52.
Shaaban ER, Shapaan M, Saddeek YB. Structural and thermal stability criteria of Bi2O3–B2O3 glasses. J Phys Condens Matter. 2008;20:155108–17.
Acknowledgment
The authors wish to thank Al-Azhar University for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aly, K.A., Dahshan, A. & Saddeek, Y.B. Effect of MoO3 additions on the thermal stability and crystallization kinetics of PbO–Sb2O3–As2O3 glasses. J Therm Anal Calorim 100, 543–549 (2010). https://doi.org/10.1007/s10973-009-0018-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0018-3