Abstract
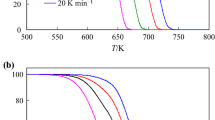
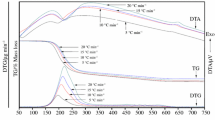
Kinetics of thermal decomposition of three structurally similar complexes Co2Cu(C2O4)3 (R-diam)2, where R is ethyl, 1,2-propyl or 1,3-propyl, was studied under non-isothermal conditions and nitrogen dynamic atmosphere at heating rates of 5, 7, 10, 12 and 15 K min−1.
For data processing the Flynn-Wall-Ozawa and a modified non-parametric kinetic methods were used. By both methods the activation energy are in the range of 97–102 kJ mol−1. The formal kinetic is r=kα(1−α)2. Also a compensation effect between lnA and E was evidenced. The kinetic analysis lead to the conclusion of an identic decomposition mechanism by a single step process.
Similar content being viewed by others
References
D. L. Trimm, Design of Industrial Catalysts, Elsevier, Amsterdam 1980.
T. Vlase, G. Vlase and N. Doca, Thermochim. Acta, 379 (2001) 59.
T. Vlase, G. Vlase and N. Doca, Thermochim. Acta, 379 (2001) 65.
T. Vlase, G. Vlase, A. Chiriac and N. Doca, J. Therm. Anal. Cal., 72 (2003) 839.
T. Vlase, G. Vlase, A. Chiriac and N. Doca, J. Therm. Anal. Cal., 72 (2003) 847.
T. Vlase, G. Vlase, A. Chiriac and N. Doca, J. Therm. Anal. Cal., 80 (2005) 87.
I. H. Flynn and L. A. Wall, Polym. Lett., 4 (1966) 323.
T. Ozawa, Bull. Chem. Soc. Jpn., 38 (1965) 1881.
M. E. Brown, et al., Thermochim. Acta, 355 (2000) 125.
R. Serra, R. Nomen and J. Sempere, J. Therm. Anal. Cal., 52 (1998) 923.
R. Serra, R. Nomen and J. Sempere, Thermochim. Acta, 316 (1998) 37.
T. Vlase, G. Vlase, N. Doca and C. Bolcu, J. Therm. Anal. Cal., 80 (2005) 59.
T. Vlase, G. Vlase and N. Doca, J. Therm. Anal. Cal., 80 (2005) 207.
T. Vlase, G. Vlase and N. Doca, J. Therm. Anal. Cal., 80 (2005) 425.
O. Costişor, C. Stănescu, S. Policec, P. Weinberger, J. Zang, and W. Linert, Orient J. Chem., 13 (1997) 197.
R. M. Silverstein, G. Clayton Basster and T. Morrell, Spectrometric Identification of the Compounds, 5th Ed., John Wiley, 1995.
M. E. Wall, et al., A Practical Approach to Microarray Data Analysis, Kluwer-Norwel, MA 2003, LNL LA-UR-02, pp. 91–109.
J. Šesták and G. Berggren, Thermochim. Acta, 3 (1971) 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vlase, G., Vlase, T., Tudose, R. et al. Kinetic of decomposition of some complexes under non-isothermal conditions. J Therm Anal Calorim 88, 637–640 (2007). https://doi.org/10.1007/s10973-006-8020-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-006-8020-5