Abstract
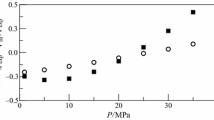
New experimental data on the density of three (0.2393, 0.4856 and 0.7390 mole fraction of ethylbenzene) binary n-heptane+ethylbenzene mixtures have been measured with a constant-volume piezometer immersed in a precision liquid thermostat. These new experimental data covering a temperature range from 306 to 527 K and a pressure range of 0.1 to 11 MPa. The experimental data reported here have an uncertainty less than 0.06% for the density, 0.05% for the pressure, 15 mK for the temperature, and 0.012% for the concentration. Excess molar volumes were derived using measured values of density for the mixtures and for the pure components calculated with reference equation of state for n-heptane (Span and Wagner, 2003) and for the pure ethylbenzene (Frenkel et al., 2005). The derived values of excess molar volumes at atmospheric pressure were compared with the values reported by other authors in the literature. The effect of pressure on the excess molar volumes was studied.
Similar content being viewed by others
References
C Díaz B Orge G Marino J Tojo (2001) J. Chem. Thermodyn. 33 1015 Occurrence Handle10.1006/jcht.2000.0830
A Qin DE Hoffman P Munk (1992) J. Chem. Eng. Data 37 61 Occurrence Handle10.1021/je00005a019 Occurrence Handle1:CAS:528:DyaK38XkslWquw%3D%3D
AM Awwad NK Al-Nidawy MA Salman FA Hassan (1987) Thermochim. Acta 114 337 Occurrence Handle10.1016/0040-6031(87)80056-4 Occurrence Handle1:CAS:528:DyaL2sXkt1yqtLo%3D
H Paul J Krug H Knapp (1986) Thermochim. Acta 108 9 Occurrence Handle10.1016/0040-6031(86)85073-0 Occurrence Handle1:CAS:528:DyaL2sXntlOgtw%3D%3D
WA Al Gherwi AH Nhaesi AA Asfour (2006) J. Sol. Chem. 35 455 Occurrence Handle10.1007/s10953-005-9005-x Occurrence Handle1:CAS:528:DC%2BD28Xlt1ansLo%3D
JPE Grolier A Faradjzadeh (1981) Int. Data Ser. Selected Data on Mixtures, Ser. A 8 140
M Cáceres Alonso JM Arsuaga Ferreras J Núnez Delgado (1985) Fluid Phase Equilib. 20 81 Occurrence Handle10.1016/0378-3812(85)90023-8
IM Abdulagatov ND Azizov (2003) J. Sol. Chem. 32 573 Occurrence Handle10.1023/A:1026388205205 Occurrence Handle1:CAS:528:DC%2BD3sXosVahsr8%3D
IM Abdulagatov ND Azizov (2003) Int. J. Thermophys. 24 1581 Occurrence Handle10.1023/B:IJOT.0000004094.08878.8e Occurrence Handle1:CAS:528:DC%2BD3sXptVCrsL8%3D
IM Abdulagatov ND Azizov (2004) J. Sol. Chem. 33 1305 Occurrence Handle10.1007/s10953-004-7142-2 Occurrence Handle1:CAS:528:DC%2BD2MXisVOrtw%3D%3D
IM Abdulagatov ND Azizov (2004) J. Chem. Thermodyn. 36 829 Occurrence Handle10.1016/j.jct.2004.06.001 Occurrence Handle1:CAS:528:DC%2BD2cXnsVGjtb0%3D
IM Abdulagatov ND Azizov (2004) Fluid Phase Equlib. 216 189 Occurrence Handle10.1016/j.fluid.2003.01.004 Occurrence Handle1:CAS:528:DC%2BD2cXotlSrtA%3D%3D
IM Abdulagatov ND Azizov (2003/2004) High Temp-High Press. 35/36 477 Occurrence Handle10.1068/htjr121 Occurrence Handle1:CAS:528:DC%2BD2MXpvFKmsrk%3D
IM Abdulagatov ND Azizov (2004) J. Chem. Thermodyn. 36 17 Occurrence Handle10.1016/j.jct.2003.09.006 Occurrence Handle1:CAS:528:DC%2BD2cXit1Wqsw%3D%3D
IM Abdulagatov ND Azizov (2006) Chem. Geology 230 22 Occurrence Handle10.1016/j.chemgeo.2005.11.010 Occurrence Handle1:CAS:528:DC%2BD28XltVOqsLg%3D
I. M. Abdulagatov and N. D. Azizov, J. Chem. Thermodyn., (2006) in press.
W Wagner A Pruß (2002) J. Phys. Chem. Ref. Data 31 387 Occurrence Handle10.1063/1.1461829 Occurrence Handle1:CAS:528:DC%2BD38Xls1OisrY%3D
FG Keyes LB Smith (1933) Proc. Amer. Acad. Arts Sci. 68 505 Occurrence Handle10.2307/20022962
R Span andW.Wagner (2003) Int. J. Thermophys. 24 41 Occurrence Handle10.1023/A:1022310214958
M Frenkel RD Chirico VV Diky X Yan Q Dong C Muzny (2005) J. Chem. Inform. Model. 45 816 Occurrence Handle10.1021/ci050067b Occurrence Handle1:CAS:528:DC%2BD2MXktF2ktbw%3D
AJ Treszczanowicz O Kiyohara GC Benson (1981) J. Chem. Thermodyn. 13 253 Occurrence Handle10.1016/0021-9614(81)90125-7 Occurrence Handle1:CAS:528:DyaL3MXkt1Cjsbg%3D
G Douheret MI Davis (1993) Chem. Soc. Rev. 22 43 Occurrence Handle10.1039/cs9932200043 Occurrence Handle1:CAS:528:DyaK3sXhsFGnu78%3D
BE de Comindges MM Pineiro E Mosteiro EM Ascato MM Mato TP Iglesias JL Legido (2002) J. Therm. Anal. Cal. 70 217 Occurrence Handle10.1023/A:1020626205538
M Cáceres Alonso J Núnez Delgado (1981) J. Chem. Thermodyn. 13 1133 Occurrence Handle10.1016/0021-9614(81)90011-2
M Cáceres Alonso J Núnez Delgado (1982) J. Chem. Eng. Data 27 331 Occurrence Handle10.1021/je00029a030
IL Acevedo L Lugo MJP Comunas EL Arancibia J Fernández (2003) J. Chem. Eng. Data 48 1271 Occurrence Handle10.1021/je034056v Occurrence Handle1:CAS:528:DC%2BD3sXls1ehu7c%3D
AK Doolittle (1964) J. Chem. Eng. Data 9 275 Occurrence Handle10.1021/je60021a048 Occurrence Handle1:CAS:528:DyaF2cXos1KrsQ%3D%3D
BM Westwood VN Kabadi (2003) J. Chem. Thermodyn. 35 1965 Occurrence Handle10.1016/j.jct.2003.08.005 Occurrence Handle1:CAS:528:DC%2BD3sXoslOgurg%3D
HR Pinnick ChL Falling GC Allred WR Parrish (1995) J. Chem. Eng. Data 40 950 Occurrence Handle10.1021/je00020a048 Occurrence Handle1:CAS:528:DyaK2MXmsVejtb0%3D
YM Naziev AN Shahverdiyev VH Hasanov (2005) J. Chem. Thermodyn. 37 1268 Occurrence Handle10.1016/j.jct.2005.03.006 Occurrence Handle1:CAS:528:DC%2BD2MXhtVamsL7L
SK Garg TS Banipal JC Ahluwalia (1993) J. Chem. Thermodyn. 25 57 Occurrence Handle10.1006/jcht.1993.1007 Occurrence Handle1:CAS:528:DyaK3sXhtFSrsLs%3D
S Malanowski R Patz MT Rätzsch C Wohlfarth (1979) Fluid Phase Equilib. 3 291 Occurrence Handle10.1016/0378-3812(79)80003-5 Occurrence Handle1:CAS:528:DyaL3cXmsFKntA%3D%3D
DVS Jain R Chadha SK Sehgal (1995) Fluid Phase Equilib. 112 151 Occurrence Handle10.1016/0378-3812(95)02791-C Occurrence Handle1:CAS:528:DyaK2MXovVyntr0%3D
Ch Yang W Yu P Ma (2005) J. Chem. Eng. Data 50 1197 Occurrence Handle10.1021/je049572f Occurrence Handle1:CAS:528:DC%2BD2MXksVSqu7o%3D
G Chen H Knapp (1995) J. Chem. Eng. Data 40 1001 Occurrence Handle10.1021/je00020a061 Occurrence Handle1:CAS:528:DyaK2MXmsVeitro%3D
M Takenaka R Tanaka S Murakami (1982) J. Chem. Thermodyn. 14 399 Occurrence Handle10.1016/0021-9614(82)90061-1 Occurrence Handle1:CAS:528:DyaL38XksVGhur8%3D
N Sastry MK Valand (1996) J. Chem. Eng. Data 41 1421 Occurrence Handle10.1021/je960135d Occurrence Handle1:CAS:528:DyaK28XmtlWqsrw%3D
Ch Berro A Peneloux (1984) J. Chem. Eng. Data 29 206 Occurrence Handle10.1021/je00036a033 Occurrence Handle1:CAS:528:DyaL2cXhsFKit7s%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdulagatov, I.M., Azizov, N.D. Volumetric properties of the binary mixture n-heptane+ethylbenzene mixtures at high temperatures and high pressures. J Therm Anal Calorim 87, 483–492 (2007). https://doi.org/10.1007/s10973-006-7906-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-006-7906-6