Abstract
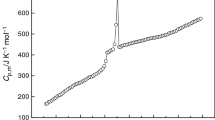
The molar heat capacities of the room temperature ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate (BMIBF4) were measured by an adiabatic calorimeter in temperature range from 80 to 390 K. The dependence of the molar heat capacity on temperature is given as a function of the reduced temperature X by polynomial equations, C P,m (J K–1 mol–1)= 195.55+47.230 X–3.1533 X 2+4.0733 X 3+3.9126 X 4 [X=(T–125.5)/45.5] for the solid phase (80~171 K), and C P,m (J K–1 mol–1)= 378.62+43.929 X+16.456 X 2–4.6684 X 3–5.5876 X 4 [X=(T–285.5)/104.5] for the liquid phase (181~390 K), respectively. According to the polynomial equations and thermodynamic relationship, the values of thermodynamic function of the BMIBF4 relative to 298.15 K were calculated in temperature range from 80 to 390 K with an interval of 5 K. The glass translation of BMIBF4 was observed at 176.24 K. Using oxygen-bomb combustion calorimeter, the molar enthalpy of combustion of BMIBF4 was determined to be Δc H m o= – 5335±17 kJ mol–1. The standard molar enthalpy of formation of BMIBF4 was evaluated to be Δf H m o= –1221.8±4.0 kJ mol–1 at T=298.150±0.001 K.
Similar content being viewed by others
References
CM Gordon JD Holbrey AR Kennedy KR Seddon (1998) J. Mater. Chem. 8 2627 Occurrence Handle1:CAS:528:DyaK1cXnvVSmsrs%3D Occurrence Handle10.1039/a806169f
J Fuller RT Cartin RA Osteryoung (1997) J. Electrochem. Soc. 144 3881 Occurrence Handle1:CAS:528:DyaK2sXnsV2nsrc%3D Occurrence Handle10.1149/1.1838106
J Sun M Forsyth DR Macfarlane (1998) J. Phys. Chem. B. 102 8858 Occurrence Handle1:CAS:528:DyaK1cXmsVWjt78%3D Occurrence Handle10.1021/jp981159p
T Welton (1999) Chem. Rev. 99 2071 Occurrence Handle1:CAS:528:DyaK1MXkt1artrw%3D Occurrence Handle10.1021/cr980032t
AJ Carmichael KR Seddon (2000) J. Phys. Org. Chem. 13 591 Occurrence Handle1:CAS:528:DC%2BD3cXns1ShsLk%3D Occurrence Handle10.1002/1099-1395(200010)13:10<591::AID-POC305>3.0.CO;2-2
CE Song WH Shim EJ Roh SG Lee JH Choi (2001) Chem. Commun. 12 1122 Occurrence Handle10.1039/b101140p Occurrence Handle1:CAS:528:DC%2BD3MXktFGqurw%3D
P Wasserscheid CM Gordon C Hilgers MJ Muldoon IR Dunkin (2001) Chem. Commun. 13 1186 Occurrence Handle10.1039/b101400p
C Wheeler KN West CL Liotta CA Eckert (2001) Chem. Commun. 10 887 Occurrence Handle10.1039/b101202a
F Endres (2001) Phys. Chem. Chem. Phys. 3 3165 Occurrence Handle1:CAS:528:DC%2BD3MXlt1ynsL8%3D Occurrence Handle10.1039/b102232f
V Najdanovic-Visak JMSS Esperanca LPN Rebelo (2002) Phys. Chem. Chem. Phys. 4 1701 Occurrence Handle1:CAS:528:DC%2BD38Xjt1yitbY%3D Occurrence Handle10.1039/b201723g
P Vasserscheid W Keim (2000) Angew. Chem. Int. Ed. 39 3772 Occurrence Handle10.1002/1521-3773(20001103)39:21<3772::AID-ANIE3772>3.0.CO;2-5
JD Holbrey KR Seddon (1999) Clean Products Processes 1 223
D Appleby CL Hussey KR Seddon JE Turp (1986) Nature 323 614 Occurrence Handle1:CAS:528:DyaL2sXktVKrsw%3D%3D Occurrence Handle10.1038/323614a0
JL Anthony FJ Maginn Brennecke (2001) J. Phys. Chem. B. 105 10942 Occurrence Handle1:CAS:528:DC%2BD3MXntl2lsLs%3D Occurrence Handle10.1021/jp0112368
JZ Yang P Tian LL He WG Xu (2003) Fluid Phase Equilib. 204 295 Occurrence Handle1:CAS:528:DC%2BD3sXhslKjsro%3D Occurrence Handle10.1016/S0378-3812(02)00265-0
JZ Yang WG Xu QG Zhang (2003) J. Chem. Thermodyn. 35 1855 Occurrence Handle1:CAS:528:DC%2BD3sXot1Sksbk%3D Occurrence Handle10.1016/j.jct.2003.07.002
JZ Yang P Tian WG Xu (2004) Thermochim. Acta 412 1 Occurrence Handle1:CAS:528:DC%2BD2cXhvV2nsb4%3D Occurrence Handle10.1016/j.tca.2003.09.008
JD Holbrey WM Reichert RP Swatloski (2002) Green Chem. 4 407 Occurrence Handle1:CAS:528:DC%2BD38XnsFequ7o%3D Occurrence Handle10.1039/b204469b
J Fuller RA Osteryoung RT Carlin (1995) J. Electrochem. Soc. 142 3632 Occurrence Handle1:CAS:528:DyaK2MXptlSmurk%3D Occurrence Handle10.1149/1.2048390
EN Jacobsen I Marko KB Sharpless (1988) J. Am. Chem. Soc. 110 1986 Occurrence Handle10.1021/ja00214a065
P Bonhote A.P Dias N Papageorgiou K Kalyanasundaram M Gratzel (1996) Inorg. Chem. 35 1168 Occurrence Handle1:CAS:528:DyaK28Xpt1Wgug%3D%3D Occurrence Handle10.1021/ic951325x
PAZ Suarez JEL Dullius S Einloft RFD Souza J Dupnot (1996) Polyhedron 157 1217 Occurrence Handle10.1016/0277-5387(95)00365-7
PJ Dyson MC Grossel N Srinivasan T Vine T Welton DJ Williams AJP White T Zigras (1997) Dalton 19 3465
RM Lau F van Rantwijk KR Seddon RA Sheldon (2000) Org. Lett. 2 4189 Occurrence Handle1:CAS:528:DC%2BD3cXotl2nsrg%3D Occurrence Handle10.1021/ol006732d
PJ Dyson MC Grossel N Srinivasan T Vine T Welton DJ Williams AJP White T Zigras (1997) J. Chem. Soc., Dalton Trans. 1 3465 Occurrence Handle10.1039/a702978k
ZC Tan GY Sun Y Sun AX Yin WB Wang JC Ye LX Zhou (1995) J. Thermal Anal. 45 59 Occurrence Handle1:CAS:528:DyaK2MXnslWmsr4%3D
ZC Tan LX Sun SH Meng L Li F Xu PYBP Liu JB Zhang (2002) J. Chem. Thermodyn. 34 1417 Occurrence Handle1:CAS:528:DC%2BD38XnsVeqsbw%3D Occurrence Handle10.1016/S0021-9614(02)00165-9
G Archer (1993) J. Phys. Chem. Ref. Data 22 1441 Occurrence Handle1:CAS:528:DyaK2cXmtlGqsg%3D%3D Occurrence Handle10.1063/1.555931
YY Di ZC Tan XH Sun MH Wang F Xu YF Liu LX Sun HT Zhang (2004) J. Chem. Thermodyn. 36 79 Occurrence Handle1:CAS:528:DC%2BD2cXmtFegsA%3D%3D Occurrence Handle10.1016/j.jct.2003.08.017
SX Wang ZC Tan YY Di F Xu MH Wang LX Sun T Zhang (2004) J. Therm. Anal. Cal. 76 335 Occurrence Handle1:CAS:528:DC%2BD2cXjvFSrs7g%3D Occurrence Handle10.1023/B:JTAN.0000027833.24442.a0
F Xu LX Sun ZC Tan J.GLiang YY Di QF Tian T Zhang. (2004) J. Therm. Anal. Cal. 76 481 Occurrence Handle1:CAS:528:DC%2BD2cXktVWhur4%3D Occurrence Handle10.1023/B:JTAN.0000028026.30886.ae
ZD Nan ZC Tan (2004) J. Therm. Anal. Cal. 76 955 Occurrence Handle1:CAS:528:DC%2BD2cXkvFGgt78%3D Occurrence Handle10.1023/B:JTAN.0000032281.40952.7e
B Xue JY Wang ZC Tan SW Lu SH Meng (2004) J. Therm. Anal. Cal. 76 965 Occurrence Handle1:CAS:528:DC%2BD2cXkvFGgtrY%3D Occurrence Handle10.1023/B:JTAN.0000032282.04071.f1
YJ Song ZC Tan SW Lu Y Xue (2004) J. Therm. Anal. Cal. 77 873 Occurrence Handle1:CAS:528:DC%2BD2cXnsFOktrs%3D Occurrence Handle10.1023/B:JTAN.0000041666.78862.90
ZC Tan B Xue SW Lu SH Meng XH Yuan YJ Song (2001) J. Therm. Anal. Cal. 63 297 Occurrence Handle1:CAS:528:DC%2BD3MXhs1Sjur4%3D Occurrence Handle10.1023/A:1010121427777
HA Skinner et al. (1962) Experimental Thermochemistry, Vol. 2 John Wiley and Sons New York, London
JD Cox DD Wagman VA Medvedev et al. (1989) CODATA Key Values for Thermodynamics Hemisphere New York
JD Cox (1978) J. Chem. Thermodyn. 10 903 Occurrence Handle1:CAS:528:DyaE1cXmt1WqsLg%3D Occurrence Handle10.1016/0021-9614(78)90050-2
M. W. Chase, Jr., NIST-JANAF Thermochemical Tables, Fourth Edition, J. Phys. Chem. Ref. Data, Monograph, 9 (1998) 1–1951.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Z.H., Tan, Z.C., Li, Y.S. et al. Thermodynamic investigation of room temperature ionic liquid. J Therm Anal Calorim 85, 551–557 (2006). https://doi.org/10.1007/s10973-006-7640-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-006-7640-0