Abstract
This work involved the synthesis of compositions of Ba0.95Ca0.05SnxTi1-xO3 (BCST) with varying amounts of Sn dopant (x = 0, 0.02, 0.04, 0.06, 0.08, and 0.1). A standard solid-state reaction approach was used to create all of the ceramic compounds. Each BCST composite’s microstructure, sintering, morphology, density, optical, and electrical characteristics were carefully examined, and the dielectric performance was optimized. In comparison to the unmodified composite, introducing varied amounts of Sn material into the BCST compound changed the crystal lattice vibrations and functional group locations. This result indicates that there are some variations in unit cell size, revealing that Sn+4 ions diffused effectively inside the lattice structure to produce BSCT composites. Further, SEM micrographs indicated proportionate changes in the homogenous structure and irregular forms as Sn concentration increased, as well as some variation in average grain size. As a consequence, by adding 0.08 mol% of Sn dopant, the crystallite size and average grain size were adjusted to 45.69 nm and 0.66 µm, respectively. Meanwhile, the 0.08-Sn specimen displayed a dielectric constant (Ɛ) with an optimum value of 5557 and a relative decrease in the Curie-Weiss constant. These results are attributed to the existence of various concentrations of Sn ions at the Ti-site of the BCT, which resulted in a compositionally disordered state. This disordered condition is essential for the production of dielectric compounds. Therefore, it is evident that modifying the amount of Sn doping added significantly enhanced the dielectric characteristics of the BCST composites created in this work. However, excessive Sn doping reduces the dielectric properties due to a reduction in tetragonal phase and an increase of disorders and charge fluctuations.
Graphical Abstract

Highlights
-
Synthesis and characterization of Ba0.95Ca0.05SnxTi1-xO3 (BCST) compositions with varying amounts of Sn dopant.
-
A standard solid-state reaction approach was used to create all of the ceramic compounds.
-
The dielectric characteristics of the BCST proved to be highly dependent on the amount of Sn doping employed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
One of the most well explored composite materials is BaTiO3 (BT), that has an ABO3 perovskite structure with barium and titanium occupying the A-site and B-site, respectively, with titanium somewhat out of the core [1, 2]. Therefore, BT compounds are a great candidate for various applications like transducers, piezoelectric sensors, multilayer capacitors, thermistors, photovoltaic components, actuators, and so on [3,4,5]. Further advancement in BT is required, and recent research is pointing in that regard [6]. As a consequence, the incorporation of calcium (Ca) into the BT lattice to produce a barium calcium titanate (BCT) molecule has lately received interest due to its higher piezoelectric sensitivity than pure BT [1, 7]. Furthermore, the microstructure, dielectric, and functional characteristics modulators may be successfully adjusted by introducing a Ca+2 approach [8, 9]. As stated before, there are two techniques to enhance the dielectric characteristics. Reducing the grain size to boost the surface-to-volume ratio is one such technique. The alternate approach [6, 10], involves replacing some of the barium in composites to affect phase boundaries. As a consequence, a lot of research has concentrated on investigating multiferroic materials, like as Sn substance based on barium titanate employed as a dopant material to create high-dielectric BCST compositions [11, 12]. Whereas, the existence of a Sn+4 ions inside the BCT matrix influenced the intrinsic and extrinsic characteristics of the ceramic compounds, such as the coexistence of phases [13], and grain size [11]. Meanwhile, it affects the Curie temperature (Tc) whenever it is consumed within its solubility limit [14]. Tc drops almost linearly with increasing Sn content. This activity is usually attributed to the small ionic size of Sn+4. Increasing the Sn level reduces the unit cell size in the perovskite crystal structure, reducing the Tc in a manner similar to that of the hydrostatic pressure [15]. Moreover, earlier studies revealed that when the doping level in BaSnxTi1-xO3 was between 0.5 and 1.5, the ferroelectric and piezoelectric characteristics of BCST composites improved significantly [16, 17]. By making reference to it, these compositions provide a promising solution for a variety of fields, including diverse linked dielectric and piezoelectric characteristics, actuators, etc. [17].
In the current work, Ba0.95Ca0.05Ti1-xSnxO3 (BCST) compositions demonstrated a sequence of phase transitions followed by variations in Sn contents (x = 0, 0.02, 0.04, 0.06, 0.08, and 0.1). The as-produced perovskites’ ultimate microstructural, sintering, morphology, phase composition, and dielectric characteristics were examined. The optimal relaxation time and the Curie-Weiss constant are then presented. Owing to the developed BCST composites’ remarkable ferroelectric properties, the dielectric constant (Ɛ) was improved via introducing Sn+4 to reach an ideal value of 5557. Additionally, BCST-based ceramics are appropriate for memory and sensing applications due to their strong relaxation characteristic.
2 Sample preparation
Traditional solid phase reaction techniques were used to incorporate Sn+4 and Ca+2 ions into the BT perovskite-type structure seen in Fig. 1. Whereas Ca+2 was added at a constant ratio of 0.05 for all synthesized composites, Sn+4 ions were mixed in various concentrations within BCT crystals to create compositions of (Ba0.95Ca0.05)(SnxTi1-x)O3 (BCST), where x = 0, 0.02, 0.04, 0.08, and 0.1. High quality oxide powders from Sigma Aldrich, including BaCO3 (99%), TiO2 (98%), CaCO3 (99.9%), and SnO2 (99.9%), were used as source ingredients and weighted according to stoichiometric ratios. After homogenizing the combined powders in an agate ball mill for 6 h, the synthesized composites were fired in the following processes. In the first fire, the resulting mixture was calcined at 1200 °C for 8 h with a heating rate of 5 °C/min. Subsequently, sifted and pressed at 20 MPa into thin discs 2 cm in diameter and 1 ~ 1.5 cm in thickness. The calcined powder was then combined with a very little amount of distilled water and pressed into discs at 40 MPa once more. These discs were also put in a small silica crucible that was in the middle of a larger crucible and coated with Aluminum oxide. Following that, the tablets received a second burning (sintering) process that lasted 3 h at 1400 °C with a heating rate of 5 °C/min. The discs were also mechanically processed to create specimens that were 1.5 mm thick and 7.5 mm in diameter. Finally, a silver substance was applied to both sides of the top and bottom electrodes of the produced BSCT discs.
3 Characterization
In order to determine the phase purity and crystal symmetries of the calcined composites produced, X-ray diffraction measurement patterns (XRD, EMMAO 143, GBC, Australia) were used in the scanning range of Bragg’s angle (2θ) 20°–80°. The Archimedes’ principle was employed to estimate the bulk density (\({{{\mathrm{\rho }}}}_{{{\mathrm{m}}}}\)) of the ceramic composites. Following that, the theoretical density (\({{{\mathrm{\rho }}}}_{{{{\mathrm{th}}}}}\)) is determined using the composition molecular weight and the weighted cell volume from the XRD measurement. In addition, FTIR spectroscopy (Jasco 4100 Japan model) was employed to evaluate changes in BCST chemical compositions as the Sn concentration increased. At ambient temperature, FTIR spectra were performed on the calcined and sintered specimens with a resolution of 4 cm–1 in the range of 4000 to 400 cm–1. Further, through using scanning electron microscopy (SEM, JOUL, JSM – 5500, Japan), the morphology of the sintered BCST was discovered. Meanwhile, a computerized SPECORD 200 PLUS analytical spectrophotometer was used to test the optical characteristics of the calcined composites with a step of 1 nm at incident light in the 360–1100 nm wavelength range. Finally, an LCR meter (E4980A, Agilent, Palo Alto, USA) of the sintered compounds was used to examine the dielectric constant, loss tangent (tanδ), and Curie Weiss characteristics.
4 Results and discussion
4.1 X-ray diffraction (XRD) Analysis
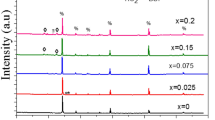
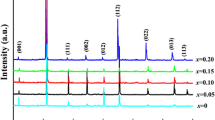
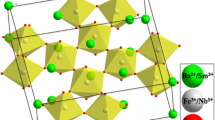
Figure 2a and b depict XRD profile peaks of as-synthesized BCSxT composites (x = 0, 0.02, 0.04, 0.06, 0.08, and 0.1) in the 20°–80° and 30°–35° ranges, respectively. All peaks in all compositions exhibit no splitting. Moreover, the detected peaks are well matched with JCPDS card no. 00-005-0626, showing that the substance crystallizes in a typical tetragonal form [16, 18]. The existence of a similar phase structure in all peaks after varying the amount of Sn+4 ions in the combinations suggests that these ions may combine with the perovskite BSCT system. Meanwhile, the deformation of the BCST lattice structure to rhombohedral phase is not observed [19]. Afterwards, to gain information into the structural evolution of the BCST compositions caused by the doping effect, the XRD data of all substances were explored using the Rietveld refinement process and analyzed using the Fullprof software [20]. Figure 3 depicts the Rietveld refinement of the BCS0.08T composite, as well as the structural model of the BCT and BCST produced by the “Vesta” software. Meanwhile, Table 1 lists the crystallographic parameters retrieved via the Rietveld refinement. It is clear that the level of Sn in the composites significantly affect the cell parameters, as ‘a’ and ‘b’ rise while ‘c’ declines as Sn concentration increases. Besides, all peaks displayed in Fig. 2a corresponding to the levels of (100), (110), (111), (200), (102), (211), (220), (212), (310) and (113) are shifted slightly towards a lower diffraction angle compared to the untreated BCST composite. Also, Fig. 2b clearly depicts this shifting behavior via utilizing a zoomed image of peak (110). The replacement of the larger ionic radius (I.R.) Sn+4 ion (I.R. = 0.69 Å) at the Ti+4-sites (I.R. = 0.605 Å) is mainly responsible for the shift of the peaks towards the lower angles [21]. However, the diffraction peaks shift slightly towards high angles with high concentrations BCS0.1T, which might be related to a structural phase transition.
According to the Bragg equation, lattice expansion causes diffraction peaks to shift to a lower angle [1]. Similarly, this trend is matched by an expansion in the lattice unit cell size of ceramics, with the level of Sn+4 ions rising to BCS0.08T as a result of ionic substitution between Sn+4 and Ti+4 in the unit cell. Whereas further increasing the dopant level causes a decline in cell volume as well as a limited shift of the diffraction peaks to higher angles, which may be attributed to partial substitution of Ba+2/Ca+2-sites by Sn+4 ions [22]. However, tetragonality (c/a) tends to decline till BCS0.04T before rising towards BCS0.08T specimen. These outcomes suggest that Sn+4 ions are well diffused inside the lattice structure and that variations in ion concentration have little effect on the composite structure. Similar behavior for BCST compounds has been reported in several previous studies [22,23,24].
On the other hand, the estimated crystallite size of BCST composites was impacted by various levels of incorporated ions [11, 17]. Scherrer’s formula was used to estimate the composites crystallite size [25]. According to Table 1, the pure compound had a size of 45.39 nm and subsequently reduced to 27.89 nm as the amount of incorporated ions increased up to Sn-0.04. Additionally, the crystallite size varied significantly with increasing Sn concentration in the perovskite lattice, with the Sn-0.08 specimen achieving an optimum value of 45.69 nm.
These differences can be explained via the energy exchange that exists between the loaded Sn+4 ions and the lattice locations of certain host atoms, consequently resulting in lattice deformation. This falls in line with the little peak-positioned shift at low Sn levels. Nevertheless, for perovskite titanate, the larger crystallites that were seen in the 0.04 ≤ Sn ≤ 0.08 samples—which also showed a similar tetragonality trend—are more desirable since they boost polarizability, thereby enhancing photoelectric characteristics [26]. Moreover, the noticed alterations suggest a greater propensity for phase transition in the BCST systems once a larger Sn dosage (e.g., 0.1-Sn) is added. As a result, a decline in the ferroelectric characteristics of the BCST matrix is predicted [27].
4.2 FTIR spectrum
FTIR spectrum was also performed in order to complete the investigation of crystal lattice vibration. As shown in Fig. 4, FTIR spectra were recorded on the as-synthesized compounds (BCSzeroT-BCS0.1T) before and after the sintering process. In the first stage before the sintering process, three major absorption bands are noticed. The first notable peak appeared between 1415–985 cm−1 and was assigned to the COO– bond. The second peak, however, is seen at 1415 cm−1, and it belongs to \({{{\mathrm{CO}}}}_3^{2 - }\) group, referring to the fact that either BaCO3 or CaCO3 or both overlap to form a single absorption peak with little shifting at high wavenumber [28]. The third one is associated to vibrations in the stretched Ti–O, Ba–O, Ca–O and Sn–O bonds and exhibits a high peak between 700–400 cm−1 [29]. Whereas, by increasing the Sn concentration, none of these oxide peaks changed in functional group locations. Following the sintering process of the second stage, some chemical bonds, like \({{{\mathrm{CO}}}}_3^{2 - }\) and COO–, disappeared [30]. The decomposition of these bonds suggests a partial carbonate ingredients reaction, which promotes the formation of the BCST phase [28]. Meanwhile, the composite’s colour gives support towards this impact via displaying colour fluctuation from grey to white with raising the processing temperature. However, a significant peak at 545 cm−1 was seen in the unaltered sample, which is attributable to Ti–O and Sn–O stretching mode [18]. The locations of these peak (545, 544, 540, 563, 574, and 580 cm−1) shifted with respect to Sn content (BCSzeroT–BCS0.1T). Whereas lower Sn concentrations caused them to move slightly in the direction of a lower wavenumber, compositions with higher concentrations exhibited the opposite trend, shifting in a higher wavenumber. Although Sn ions have a higher atomic mass than Ti ions (118.71 amu vs. 47.867 amu), less energy is needed to extend the vibration of TiO6 than SnO6 [31]. Therefore, the peak in the BCS0.02T and BCS0.04T compounds exhibited a slightly shift to a lower energy than that found for the BCSzeroT compound, indicating the existence of Sn+4 ions with a small concentrations will not be able to displace Ti+4 ions from its position, however will be able to penetrate the crystal at the same site [24]. Meanwhile, In concentrated BCST (x ≥ 0.06) compounds, the peak moves to higher energy compared to that reported in the BCSzeroT, indicates the presence of Sn+4 ion at the Ti+4 ion site of barium titanate [32, 33]. Also, the presence of minor peaks in the range of 3000–3500 cm−1 suggests the entrance of potassium bromide.
4.3 Surface morphology and density measurement
The surface micrographs of the as-synthesized BCST composites are displayed in Fig. 5a–f. The pellets in the acquired micrographs have a mostly uniform structure and relatively irregular shapes [34]. Further, employing analytical methods, the statistics of the average grain size, bulk density, and relative densities of each were investigated, and the results are listed in Table 2. The small amounts of incorporated ions (0 ≤ x ≤ 0.04), will not be able to displace Ti+4 ions from its position, however will be able to penetrate the crystal at the same site [24]. This re-positioning action, along with increased lattice strain energy, leads to reduced grain growth in treated BCST. Additionally, the non-uniform distribution of added ions in grain boundary regions also restricts grains expansion, by preventing grain boundaries from moving and inhibiting mass transit [35]. However, further addition of Sn+4 (x ≥ 0.06) resulted in the opposite behavior, as the average grain size grew and reached an optimal value of 0.66 m at the 0.08-Sn dopant. These findings indicate that Sn+4 ions grow independently of Ti+4 ions inside their own lattice, resulting in a decline in the quantity of Bi+3 in the matrix and a rise in the average grain size of BCST [36, 37]. The typical grain sizes morphology observed in SEM investigations are larger than the estimated crystallite sizes derived from the Debye-Scherrer equation (XRD). Differences in crystallite and grain size are mostly due to the formation process; crystallite size is a measure of the material’s microstructure, while grain size is a measure of its macrostructure [38]. Whereas, crystallite size is the average size of individual crystalline domains in the material, whilst grain size represents the average size of grains or grains of similar crystalline size [38]. In general, grains are made up of crystallites, and both grain and crystallite are single crystals. Given the nucleation and growth kinetics, smaller crystallites get closer and expand to grow larger during processing [39]. The completely monocrystallized portion of a grain is called the grain size, while the size of the crystallites on a specific plane within a crystal structure is called the crystallite size [39]. Many variables influence this disparity, including the kind of material, temperature sensitivity, melting temperatures, crystalline domains, and grains. Therefore, in the most likely situation, the grain is larger than a crystallite size [40]. Our results indicate that every grain seen by SEM is composed of several crystallites.
On the other hand, the influence of Sn content on composites microstructure and average grain size resulted in a corresponding variation in the bulk and relative density of the BCST compositions, given in Table 2. Whereas, the values of bulk density (\({{{\mathrm{\rho }}}}_{{{\mathrm{m}}}}\)) and relative density (\({{{\mathrm{\rho }}}}_{{{{\mathrm{relative}}}}}\)) were evaluated using the theoretical density (\({{{\mathrm{\rho }}}}_{{{{\mathrm{th}}}}}\)). Meanwhile, \({{{\mathrm{\rho }}}}_{{{{\mathrm{th}}}}}\) was calculated from the composition molecular weight and weighted unit cell volume received from XRD measurement. The relative densities were estimated using the formula below [41]:
For low dopant concentrations, the bulk density declines, extending from 5.61 g/cm3 (95.86% of its TD) for BCSzeroT to 5.45 g/cm3 (93.05% of its TD) for BCS0.04T composite. The relatively small concentrations of integrated Sn+2 ions may penetrate the crystal at the same location as the Ti+4 ions, but they cannot move them from their position [24]. Reduced density might result from this partially shifting action and greater lattice strain energy, leading in a slower Ion diffusion process during sintering and thus reducing the density expansion [35, 42]. However, there are rises in concentrated compounds (x ≥ 0.06), reaching an optimal value of 5.68 g/cm3 (97.77% of its TD) for BCS0.08T, as confirmed by the dense microstructure detected in the SEM image. These improvements in density are related to the formation of finer particles which in turn stimulate densification by growing atomic diffusion process rates, resulting in denser microstructures [43]. Since Ti+4 at B-sites was replaced by Sn+4 with smaller ionic radii and consequently there is a decline in Ti–O bonds, the bonding between Sn+4 and the TiO6 octahedral structure gets weaker, the bulk density of doped BCST ceramic material grows as the Sn ratio rises. Numerous studies have been carried out with this response [21, 42, 44]. The deviation of Sn+4 ions is simpler at B-sites due to the presence of additional space, thus enhancing ferroelectricity and elevating dielectric behavior [44]. Additionally, the Sn ion activation energies coupled to the ionization of oxygen vacancies complicate electric domain rotation, boosting the combinations’ dielectric performance [19]. Following that, a drop in density was reported to 5.59 g/cm3 (96.18% of its TD) for the heavily doped BCS0.1T system as a result to increase coarsening, which might be related to a structural phase transition.
The aforementioned transition from densification to coarsening is the result of the possibility of agglomerates forming when particle size is reduced. This agglomeration behaviour, according to a well-known fact, resulted from a reducing initial particle size that grew in its specific surface area and, therefore, its surface free energy which serves as the driving force for sintering [43, 45]. In addition, this behaviour grows increasingly prevalent as particle size falls due to the dominance of electrostatic and other surface forces that emerge with growing surface/volume ratio while particle size reduces [46, 47]. Besides, the tendency of these agglomerates to sinter together after heating causes the microstructure to become coarser.
4.4 Optical properties
A representative absorbance spectrum of the as-prepared BCST compounds is exposed in Fig. 6a. In the wavelength range of 400–900 nm, the unmodified composite exhibits a slight variation in absorption inclination, however lower 400 nm, a high absorption is seen. Additionally, Sn doped BCST compounds exhibit extra relative absorption inclination in the same region in their absorption pattern. The higher absorption of light in the visible spectrum is indicated by this inclination. This behavior is consistent with the literature findings [48]. Besides, Tauc’s relationship was employed to determine the energy band gap (Eg) for all compositions [49, 50]. According to Fig. 6b, the estimated Eg values were significantly impacted by the Sn incorporation process within the BCST matrix. Whereas, it begins at 4.66 eV for the unmodified compound and slowly reduces in respect to the Sn level, reaching its lowest value of 4.26 eV at Sn-0.08 specimen. These differences in the results for Eg could be explained by the existence of intermediary energy levels within the structure of Eg. These intermediary levels relate to the amount of structural order-disorder present on the lattice as a result of the symmetry break between the bonds of O–Sn–O and O–Ti–O and/or distortion on the groups of Sn+4 and Ti+4 [51, 52]. According to Cavalcante et al. [51], the presence of deep holes is related to low Eg values, whereas shallow holes are linked to high Eg values. Therefore, the broad band gap ~4.7 eV recorded at 0.1-Sn may be a result of the band gap’s shallow holes. Trend can draw the conclusion that adding Sn dopant ions to BCST systems can modify the optical characteristics of the material generating an active ceramic composite.
4.5 Dielectric properties of BCST composites
It is noteworthy that the dielectric constant (ℇ) value is significantly influenced by the dopant type, dopant ratio, and sintering temperatures. Additionally, there are other both intrinsic and external elements that can be affecting, including grain size, grain boundaries, density, pressure, and domain wall motion. Figure 7a depicts the temperature-dependent dielectric constant at various Sn concentrations. The ferroelectric–paraelectric phase transition, which establishes the Curie temperature, is thought to be responsible for a little hunch in the observed dielectric constant spectra in all compounds [53]. The dielectric characteristics of BCST systems are driven by this ferroelectric distortion. Since the electric dipoles freeze at relatively low temperature, ℇ appears small. In contrast, raising the temperature resulted in a rise in ℇ values, which is ascribed to the thermally stimulated relaxation process [2]. Based on a structural perspective, adding Sn+2 as a doping substance could impact the properties of the BCT matrix since Sn+2 and Ba+2 possess ionic radii of 146 pm and 118 pm, respectively. However, during the temperature rise, a portion of Sn+2 was oxidized to Sn+4, resulting in currently acting as an effective substituent for B-site [54]. At low Sn levels, the off-center movement of A and B location atoms rises dramatically when Sn+4 substitutes Ti+4 to generate deformed cubic BCST owing to a shift in cationic and anionic hybridization. In light of this, it is anticipated that the dielectric characteristics of BCST composites would significantly enhance in comparison to basic BCT [55]. As the Sn ratio grew, some flaws that resulted in oxygen vacancies were steered towards structural transitions. With respect to its high solubility, equal partial replacement of Ti+4 can lead to durable, dense binary BCST ceramic [56]. Sn doping produced oxygen vacancies, whereby could be thermally ionized at higher temperatures. Given of the vacancies’ mobility, greater vacancy level, and increased oxygen ion inertia, conduction of the vacancies would eventually gain dominant. Besides, it is most likely the nearby double ionized oxygen vacancy that provides the dipoles that supports the aliovalent Sn+4 material. The dipole orientations were influenced by the ions’ random arrangement inside the specific unit cells. Nevertheless, the arrangement the aforementioned dipoles resulted in the nucleation of polar nano-regions on a local level on the nanoscale. A higher dielectric permittivity value would ultimately follow from this [57]. Therefore, doping-induced crystal flaws are the cause of the doped specimen’s increased dielectric permittivity. Kim et al. [58] suggested that Sn-doping BT might be advantageous for outstanding stability and dependability in capacitor applications.
Notably, the presence of saturated Sn dopant levels revealed the ferroelectric phase of BCST. Nevertheless, more B-sublattice doping resulted in a decreased dipole movement owing to the phase shift that accompanied the electrical conductivity contribution. Additionally, the decreased net polarization values recorded for BCST matrix suggest that the growing structural disorder contributes to the ferroelectric ordering to decline and a relaxor-like state to arise. Moreover, the degree of t scale was identified to be in line with the tetragonal, cubic, and orthorhombic tendencies in the perovskite phase-stable series. As a consequence, a smaller t would indicate a tetragonal phase with less stability. Therefore, one would notice a reduction in the TC from the tetragonal ferroelectric phase to the cubic paraelectric phase [59]. Pavithra et al. [28], Srikanth et al. [60], and Sharma et al. [61] displayed similar transitions for BCT–BST, BCT–BSTO3, and BCT–VO3 compositions, respectively. Meanwhile, Fig. 7b depicts the Debye’s relaxation time (τ) behavior of the BCST systems as a function of temperature. The following formula was also used to estimate the values of the Debye relaxation time [62].
Where \(\varepsilon \prime\) represents the dielectric constant at angular frequency \(\omega\), \(\varepsilon _0\) and \(\varepsilon _\infty\) denotes the dielectric constants of low and infinite frequency, respectively, and \(\tau\) is the mean relaxation time [63]. For low Sn contents (x ≥ 0.04), the Debye relaxation time (τ) appears to be small (Fig. 7b). However, increasing the level of the Sn dopant (x = 0.06) resulted in a rise in values, which was attributed to increased defects, vacancy, and disordered sites within the composites’ structural system. Whereas, Substitution of Sn+4 ions at the Ti+4 site results in a substantially lower overlap between Sn+4 and O–2 than between Ti+4 and O–2. Therefore, dipole formation might be decreased, thus reducing interacting between continuous dipoles. Hence, the growth of polar nanoregions is induced by incorporating of Sn at a relatively high level, strengthening the relaxation behaviour [64, 65]. On the other hand, the highly concentrated BCST ceramic systems (x = 0.08, x = 0.1) achieved the lowest values of τ. Characteristics of the relaxation time distribution in the paraelectric—ferroelectric phase transition range suggested the existence of a “order-disorder” phenomenon during the phase transition [66]. This broader variation in temperature of the dielectric susceptibility at the phase transition vicinity—typical of structurally disordered materials—corresponded to the lower relaxation times. It is noteworthy that these outcomes match the Lyddane-Sachs-Teller relationship [67]. Given the strong concordance between this behavior of τ and its opposite behaviour for ℇ, the domain size is proportional to τ [28]. Therefore, this performance is essential for evaluating the findings of based on the critical C for ferroelectric substantial. The obtained data clearly reveal that the compound with an 0.08-Sn content had an optimal ℇ of 5557, whereas τ displayed a value of about 2.96 × 10−5 sec.
Accordingly, this pattern was supported by the measured dielectric loss values (tanδ, 1 kHz) at 27 °C. Depending on the Sn level in each composition, x = 0, 0.02, 0.04, 0.06, the reported values of tanδ were 0.0281, 0.0242, 0.0261, 0.0265, respectively, while yielding a minimum value of 0.0223 for the 0.08-Sn sample. Meanwhile, the additional quantity of filler x = 0.1 within the BCST system got 0.0253 of the tanδ value. Moreover, this dielectric loss sequence corresponds to the density improvement noticed with modifications to Sn concentration. Whenever a BCS0.08T specimen is sintered at a sufficient temperature, the microstructure coarsens, allowing agglomerates to grow. These agglomerates reduce the density of conduction paths for free mobile electrons, therefore raising the dielectric constant value.
In Table 3, the optimal dielectric value obtained for the BCS0.08T system were compared with that in previous literature research. With the same production technique and doping substances, the as-prepared BCST composition had a relatively high dielectric constant value of 5557, demonstrating excellent Sn/Ca concentration ratio performance in terms of dielectric constant.
Figure 8 depicts the variation in Curie temperature or Tc (ferroelectric transition temperature) and the maximum value of the dielectric constant (ℇm) as functions of the Sn levels inside the BCST structure. With an alteration rate of 10 °C every 2 mol%, the Tc variations almost clearly showed a linear trend. The principal reason of these variations in ℇm values is grain size, specifically differences in grain number and grain borders. This causes a large shift in Lorentz fields [68, 69]. For more details, adding a small amount of 0.02-Sn would be unable to displace the Ti+4 out of its position. As a consequence, it penetrates the crystal in the same location as it and increases the ℇm value relative to an untreated one. Despite the fact that the average grain size increases when the Sn+4 concentration at the Ti+4 position increases [70], BCTS-0.04 has both a smaller average grain size and a smaller unit cell. As a result, the dielectric constant similarly reduces until it reaches its lowest level at ℇm = 2479. This reduction in grain size when Sn+4 is replaced at the Ti+4 position is ascribed to the restriction of grain expansion caused by the presence of oxygen vacancies formed by charge compensation process linked with substitution of higher valence Sn+4 with lower valence Ba+2 is comparable to altering in (Ba1-xCax) TiO3 [71,72,73]. Following that, the Sn+4 level grows and exists independently of the Ti+4 ions within its own lattice. In this instance, compositional variations enhance the ℇm value, which reaches its greatest value at 0.08-Sn specimen. Additionally, at 0.08-Sn, the dielectric peak width seems to be broader, indicating the performance of the diffusion phase transition. This expansion of the dielectric peak is caused by changes in the ceramics’ polarization or spatial arrangement [74]. However, the continued increase in the Sn content has led to a reduce in the ℇm value, may be attributed to the following reasons:
-
Because of the growing disordered structure, lower temperatures were needed to overcome the barrier to domain shifting and domain wall motion [75].
-
lowering the connection between ferroelectric dipoles or the ferroelectric energy [76].
-
By increasing the site disorder and charge fluctuation at x > 0.08 Sn, the thermal induced current is reduced [77].
Figure 9 exposed how varying Sn concentrations affect the modified BCST compounds’ degree of relaxation (γ) values. Additionally, the dielectric performance with diffuse phase transitions was represented using the Curie-Weiss law (2) relation as follows:
Where ℇm is the highest dielectric constant at Tc, T is the absolute temperature, γ is the diffusion coefficient, and C is the Curie-Weiss constant [28, 78, 79]. When the value of γ is 1, the composite exhibits a typical ferroelectric phase transition and behaves normally according to the Curie-Weiss equation. Besides, compositions are said to be diffuse phase transition materials if γ is 2 [80]. Low dopant levels in the BCST lattice produced these high magnitudes of γ = 1.871 and γ = 1.79 with 0.02-Sn and 0.04-Sn, respectively, while the high Sn ratios induced a rapid decline. Given Ti sublattice doping, there was a shift in grain size (about one order of magnitude) and a gain in porosity, which suppressed ferroelectric characteristics and even induced a relaxor-like condition (Sn doping). In addition, the loading of Sn ions influenced the electric susceptibility anomaly associated with the presence of the low-temperature ferroelectric–paraelectric phase transition [59]. Subsequently was additionally evaluated for a tendency for relaxor-like qualities to arise. In light of the relaxor behavior for these ceramics, the transition temperature changes, the deviation from the irregular first-order ferroelectric–paraelectric transition, plus the additional propagation of this transition were modified. However, the lower γ values at concentrated ceramics revealed that the growing defects in structure contributed to the ferroelectric ordering to weaken along with a relaxor-like state to develop. Szeremeta et al. [59] highlighted similar behavior for modified-BCT ceramics. In this vein, the Curie-Weiss constant rose (3.14 × 104 °C – 5.8 × 104 °C) with increasing Sn ion concentration (x = 0–0.06), it declined to 1.13 × 104 °C in the 0.08-Sn sample. This drop in the aforementioned value could be ascribed to a reduced diffusiveness at the phase transition temperature. Additionally, this suggests that Sn ions are partially replacing at the Ti position, which assists in organizing the dipoles and reduces the variety of Curie points [61]. These finding suggest that BCST compounds exhibit clear diffuse phase transitions and high-grade ferroelectric ceramics [81].
5 Conclusion
Polycrystalline Ba0.95Ca0.05SnxTi1-xO3 (BCST) composites were successfully produced with different levels of Sn dopant (x = 0, 0.02, 0.04, 0.06, 0.08, and 0.1) using a typical solid-state reaction approach. Herein, the work focused on the influence of Ca/Sn substitution on the optical, morphological and dielectric properties of pure BT. SEM micrographs revealed some fluctuation in average grain size as well as corresponding changes in the homogeneous structure and irregular shapes as Sn concentration increased. As a result, the average grain size and crystallite size were optimized to 45.69 nm and 0.66 µm, respectively. Where these results show the existence of Sn+4 ions at the Ti+4-site and the preservation of the perovskite tetragonal structure. The 0.08-Sn specimen, however, showed a relative reduction in the Curie-Weiss constant and a dielectric constant with an ideal value of 5557. These factors indicate that BCST ceramics exhibit clear diffuse phase transitions and high-grade dielectric materials. Therefore, it is obvious that varying the level of Sn doping applied considerably enhanced the dielectric characteristics of the BCST composites created in this study.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Li L-Y, Tang X-G (2009) Effect of electric field on the dielectric properties and ferroelectric phase transition of sol–gel derived (Ba0. 90Ca0. 10) TiO3 ceramics. Mater Chem Phys 115(2–3):507–511.
Arya E et al. (2022) “Structural, dielectric and magnetic properties of Ca doped barium hexaferrite-Na0. 5Bi0. 5TiO3 magneto-electric composites,”. Mater Today Proc 82:145–150. https://doi.org/10.1016/j.matpr.2022.12.112.
Auromun K, Acharya T, Choudhary RNP (2022) Octahedral distortion-driven phase transition, relaxation and conduction process of Zr/Sn modified barium titanate relaxors. Ceram Int 48(14):20858–20871.
Yadagiri K, et al. (2023) Terahertz based optical & electrical properties of PZT. J Sol-Gel Sci Technol 1–9. https://doi.org/10.1007/s10971-023-06262-z.
Gupta KK, Tan C-P, Hsu C-M, Lin B-C, Lu C-H et al. (2023) Bio-polymer agar-assisted sol-gel synthesis and electrochemical characterization of bi-pyramidal LiNi0. 8Co0. 1Mn0. 1O2 cathode materials for lithium-ion batteries. J Sol-Gel Sci Technol 108:685–694. https://doi.org/10.1007/s10971-023-06212-9.
Yue Q, Luo L, Jiang X, Li W, Zhou J (2014) Aging effect of Mn-doped Ba0. 77Ca0. 23TiO3 ceramics. J Alloy Compd 610:276–280.
Zhang J, Dai J-Q, Lu Y-S (2023) Effect of Mg and Mn co-doping on the high electrical properties of BiFe1-2xMgxMnxO3− BaTiO3 lead-free ceramics prepared by sol–gel method and two-step sintering method. J Sol-Gel Sci Technol 106(3):804–815.
Zheng T et al. (2021) Piezoelectric calcium/manganese-doped barium titanate nanofibers with improved osteogenic activity. Ceram Int 47(20):28778–28789.
Gong G et al. (2023) Magnetodielectric coupling in Co/Mn compensated Ca3Co1. x Mn1-x O6 compounds. J Sol-Gel Sci Technol 108:791–797. https://doi.org/10.1007/s10971-023-06235-2.
Mao W et al. (2019) Combined experimental and theoretical investigation on modulation of multiferroic properties in BiFeO3 ceramics induced by Dy and transition metals co-doping. J Alloy Compd 784:117–124.
Selvaraj M, Venkatesan R, Mayandi J, Venkatachalapathy V (2021) Influence of tin (IV) doping on structural and optical properties of rhombohedral barium titanate (BaTiO3). Mater Today Proc 35:13–16.
Kumar S, Luthra V (2021) Raman and infrared spectroscopic investigation of the effects of yttrium and tin co-doping in barium titanate. J Phys Chem Solids, 154:110079.
Chen Z, Li Z, Ding J, Qiu J, Yang Y (2017) Piezoelectric and ferroelectric properties of Ba0. 9Ca0. 1Ti0. 9Sn0. 1O3 lead-free ceramics with La2O3 addition. J Alloy Compd 704:193–196.
Jaffe WR (1971) Cook, and H. Jaffe, Piezoelectric Ceramics. Academic Press, New York.
Frayssignes H, Cheng BL, Fantozzi G, Button TW (2005) Phase transformation in BST ceramics investigated by internal friction measurements. J Eur Ceram Soc 25(13):3203–3206.
Chen X et al. (2019) Strongly enhanced ferroelectric performance in Ca-doped barium titanate coatings produced by plasma electrolytic oxidation. Ceram Int 45(10):13024–13029.
Drdlik D, Marak V, Maca K, Drdlikova K (2022) Modification of barium titanate sintering via rare earth oxides addition: Dilatometric and microstructural study. Ceram Int 48(17):24599–24608.
Rached A et al. (2020) Effect of doping in the physico-chemical properties of BaTiO3 ceramics. Phys B Condens Matter 596:412343.
Mahajan A, Zhang H, Wu J, Ramana V E, Reece MJ, Yan H (2017) Effect of phase transitions on thermal depoling in lead-free 0.94 (Bi0. 5Na0. 5TiO3)–0.06 (BaTiO3) based piezoelectrics. J Phys Chem C 121(10):5709–5718.
Rodriguez-Carvajal J, “Laboratory,” FULLPROF, a Rietveld pattern matching Anal. program, Lab. Leon Brillouin, CEA-CNRS, Fr. [https://www.ill.eu/sites/fullprof/], version July, 2011.
Singh KC, Nath AK, Laishram R, Thakur OP (2011) Structural, electrical and piezoelectric properties of nanocrystalline tin-substituted barium titanate ceramics. J Alloy Compd, 509(5):2597–2601.
Ramovatar, Coondoo I, Satapathy S, Panwar N (2019) Impact of tin substitution on the structural, dielectric, ferroelectric and piezoelectric properties of Ba0. 98Ca0. 02TiO3 ceramics. Phys B Condens Matter 553:68–75. https://doi.org/10.1016/j.physb.2018.10.039.
hui Chen Z et al. (2017) Improvement on electrical properties of Ba0.9Ca0.1Ti0.9Sn0.1O3 ceramics with the addition of CeO2. J Alloy Compd 720:562–566. https://doi.org/10.1016/j.jallcom.2017.05.220.
Chen Y, Fan H, Hou D, Jia Y, Zhang A, Wang W (2022) Bismuth sodium titanate-barium titanate-barium zirconate titanate relaxor ferroelectric ceramics with high recoverable energy storage density. Ceram Int 48(18):26894–26903.
Wilson AJC (1961) X-ray Metallography by A. Taylor. International Union of Crystallography. Acta Cryst 14:696. https://doi.org/10.1107/S0365110X61004010.
Mady HA (2011) XRD and electric properties of lead barium titanate ferroelectric ceramic. Austral J Basic Appl Sci 5(10):1472–1477.
Ahmadu U, Soje AA, Usman AB, Musa AM, Isaha KU (2016) Moreover, the noticed alterations suggest a greater propensity for phase transition in the BCST systems once a larger Sn dosage (e.g., 0.1-Sn) is added. As a result, a decline in the ferroelectric characteristics of the BCST ceramics is predicted. Process Appl Ceram 10(2):79–85. https://doi.org/10.2298/PAC1602079A.
Pavithra C, Madhuri W, Kiran SR (2021) Effects of synthesis and sintering temperature in BCT-BST ceramics. Mater Chem Phys 258:123921.
Manohar A, Krishnamoorthi C (2018) Structural, Raman, magnetic and other properties of co-substituted ZnFe2O4 nanocrystals synthesized by solvothermal reflux method. J Mater Sci Mater Electron 29:737–745.
Zhu Z et al. (2015) Ferroelectric and piezoelectric properties of Ba (Ti0. 89Sn0. 11) O3 thin films prepared by sol–gel method. Chem Phys Lett 638:168–172.
Ansaree MJ, Upadhyay S (2017) Study of phase evolution and dielectric properties of Sn-doped barium titanate. Emerg Mater Res 6(1):21–28.
Kumari US, Suresh P, Rao AVP (2013) Solid-state metathetic synthesis of phase pure BaSnO3 and BaZrO3. Int Res J Pure Appl Chem 3(4):347.
Lu W, Schmidt H (2007) Preparation and characterization of BaSnO 3 powders by hydrothermal synthesis from tin oxide hydrate gel. J Mater Sci 42:10007–10013.
Wang X et al. (2021) B-site acceptor doped AgNbO3 lead-free antiferroelectric ceramics: the role of dopant on microstructure and breakdown strength. Ceram Int 47(3):3699–3705.
Lin D, Kwok KW, Chan HLW (2007) Effects of BaO on the structure and electrical properties of 0.95 K0. 5Na0. 5 (Nb0. 94Sb0. 06) O3–0.05 LiTaO3 lead-free ceramics. J Phys D Appl Phys 40(21):6778.
Wang M et al. (2021) Ultrahigh energy storage density and efficiency in Bi0. 5Na0. 5TiO3-based ceramics via the domain and bandgap engineering. ACS Appl Mater Interfaces 13(43):51218–51229.
Luo C et al. (2022) Significantly enhanced energy-storage properties of Bi0. 47Na0. 47Ba0. 06TiO3-CaHfO3 ceramics by introducing Sr0. 7Bi0. 2TiO3 for pulse capacitor application. Chem Eng J 429:132165.
Lopez Maldonado KL et al. (2014) Effects of grain boundary width and crystallite size on conductivity and magnetic properties of magnetite nanoparticles. J Nanopart Res 16:1–12.
Rhouma FIH, Dhahri A, Dhahri J, Belmabrouk H, Valente MA (2012) Structural and dielectric properties of Ba0. 8 La0. 133 Ti0. 90 Sn0. 1 O3. Solid State Commun 152(20):1874–1879.
McNaught AD, Wilkinson A (1997) Compendium of chemical terminology, vol. 1669. Blackwell Science Oxford.
Puli VS, Kumar A, Chrisey DB, Tomozawa M, Scott JF, Katiyar RS (2011) Barium zirconate-titanate/barium calcium-titanate ceramics via sol–gel process: novel high-energy-density capacitors. J Phys D Appl Phys 44(39):395403.
Liang Y et al. (2021) A tunable B-site doping SBT-BNT-SMN ceramic composite with high recoverable energy density and temperature stability. J Mater Sci 56:19564–19576.
Chitra, Laishram R, Rajput S, Singh KC (2020) Particle-size-induced high piezoelectricity in (Ba0.88Ca0.12)(Ti0.94Sn0.06)O3 piezoceramics prepared from nanopowders. J Alloys Compd. 812:152128. https://doi.org/10.1016/j.jallcom.2019.152128.
Chitra, Laishram R, Vashishtha A, Singh MK, Chandra KK, Singh KC (2019) Effect of holmium doping on structural, electrical and piezoelectric properties of lead-free (Ba,Ca)(Ti,Sn)O 3 ceramics. J Mater Sci Mater Electron 30(4):3965–3972. https://doi.org/10.1007/s10854-019-00682-w.
Zuo R, Rödel J, Chen R, Li L (2006) Sintering and electrical properties of lead‐free Na0. 5K0. 5NbO3 piezoelectric ceramics. J Am Ceram Soc 89(6):2010–2015.
Chitra, Rawat S, Agarwal S, Singh KC (2022) Lead-free (Ba0.88Ca0.12)(Ti0.94Sn0.06)O3 piezoceramics: A comprehensive analysis of the phase evolution and enhancement of electrical properties induced by high energy ball milling. Mater Chem Phys 279:125735. https://doi.org/10.1016/j.matchemphys.2022.125735.
RA Ryntz RA, Yaneff PV (2003) Coatings of polymers and plastics, New York: Marcel Dekker, CRC Press, vol. 21, p 416.
Srikanth KS, Hooda MK, Singh H, Singh VP, Vaish R (2018) Structural and photocatalytic performance of (Ba, Ca) TiO3–Ba (Sn, Ti) O3 ferroelectric ceramics. Mater Sci Semicond Process 79:153–160.
Pankove JI (1975) Optical processes in semiconductors. Dover Publications Inc. Courier Corporation, New York, p 422.
Zak AK, Abd WH (2013) Majid, MR Mahmoudian, M. Darroudi and R. Yousefi. Adv Powder Technol 24:618–624.
Cavalcante LS et al. (2009) Microstructure, dielectric properties and optical band gap control on the photoluminescence behavior of Ba [Zr 0.25 Ti 0.75] O3 thin films. J Sol-Gel Sci Technol 49:35–46.
Sczancoski JC et al. (2010) Structure and optical properties of [Ba1–xY2x/3](Zr0. 25Ti0. 75) O3 powders. Solid State Sci 12(7):1160–1167.
Zhang H, Xu P, Patterson E, Zang J, Jiang S, Rödel J (2015) Preparation and enhanced electrical properties of grain-oriented (Bi1/2Na1/2) TiO3-based lead-free incipient piezoceramics. J Eur Ceram Soc 35(9):2501–2512.
Tomar R, Naaz T, Pandit S, Mathuriya AS, Jadhav DA (2024) Exploring the potential of metal-doped perovskite-oxides as oxygen reduction catalyst for enhancing the performance of microbial desalination cells. Fuel 356:129451. https://doi.org/10.1016/j.fuel.2023.129451.
Dutta S et al. (2023) Enhancement of dielectric properties and conduction mechanism in BaTi0.85Sn0.15O3 for energy storage application. J Energy Storage 72:109335. https://doi.org/10.1016/j.est.2023.109335.
Ansari MA, Sreenivas K (2019) Effects of disorder activated scattering and defect-induced phase on the ferroelectric properties of BaSnxTi1-xO3 (0≤ x≤ 0.28) ceramics. Ceram Int 45(16):20738–20749.
Tomar R, Pandey R, Singh NB, Gupta MK, Gupta P (2020) Electrical properties of barium titanate in presence of Sn 2+ dopant. SN Appl Sci 2:1–7.
Kim TY et al. (2021) (111)-oriented Sn-doped BaTiO3 epitaxial thin films for ultrahigh energy density capacitors. Ceram Int. 47(19):26856–26862.
Szeremeta AZ et al. (2023) Doping influence on structural ferroelectric phase transitions and electrical features of barium calcium titanate. J Eur Ceram Soc 43(9):4029–4043. https://doi.org/10.1016/j.jeurceramsoc.2023.03.018.
Srikanth KS, Patel S, Steiner S, Vaish R (2018) Pyroelectric signals in (Ba, Ca) TiO3-xBa (Sn, Ti) O3 ceramics: a viable alternative for lead-based ceramics. Scr Mater 146:146–149.
Sharma P, Berwal N, Ahlawat N, Maan AS, Punia R (2019) Study of structural, dielectric, ferroelectric and magnetic properties of vanadium doped BCT ceramics. Ceram Int 45(16):20368–20378.
Hench LL, West JK (1990) Principles of Electronic Ceramics. John Wiley & Sons, New York.
Verma K, Sharma S (2012) Impedance spectroscopy and dielectric behavior in barium strontium titanate-nickel zinc ferrite composites. Phys Status Solidi Basic Res 249(1):209–216. https://doi.org/10.1002/pssb.201147322.
Sun N, Li Y, Zhang Q, Hao X (2018) Giant energy-storage density and high efficiency achieved in (Bi 0.5 Na 0.5) TiO 3–Bi (Ni 0.5 Zr 0.5) O3 thick films with polar nanoregions. J Mater Chem C 6(40):10693–10703.
Liu G et al. (2020) Structure evolution, ferroelectric properties, and energy storage performance of CaSnO3 modified BaTiO3-based Pb-free ceramics J Alloys Compd 826 https://doi.org/10.1016/j.jallcom.2020.154160.
Grigas J (2019) Microwave dielectric spectroscopy of ferroelectrics and related materials. CRC Press.
Sievers AJ, Page JB (1990) Generalized Lyddane-Sachs-Teller relation and disordered solids. Phys Rev B 41(6):3455.
Gu HH, Chen XM, Qin N (2008) Tunable dielectric characteristics of (Ba 0.95 Ca 0.05)(Ti 1− y Sn y) O 3 ferroelectric ceramics. J Electroceram 21:495–498.
Pavithra C, Madhuri W (2018) Dielectric, piezo and ferroelectric properties of microwave sintered PbTiO3 synthesized by sol–gel method. J Sol-Gel Sci Technol 85:437–445.
Elorika P, Jayasri S, Sharmistha A, Anwar S (2022) Synthesis conditions induced disorder and its role in affecting structural, dielectric, piezoelectric, optical behavior and enhancing energy storage efficiency in (Ba1-xCax) TiO3 ceramics. Ceram Int 48(13):19324–19335.
Park JG, Oh TS, Kim YH (1992) Dielectric properties and microstructural behaviour of B-site calcium-doped barium titanate ceramics J Mater Sci 27:5713–5719
Panigrahi MR, Panigrahi S (2010) Diffuse phase transition and dielectric study in Ba0. 95Ca0. 05TiO3 ceramic. Phys B Condens Matter 405(11):2556–2559.
Zaman T, Islam MK, Rahman MA, Hussain A, Matin MA, Rahman MS (2019) Mono and co-substitution of Sr2+ and Ca2+ on the structural, electrical and optical properties of barium titanate ceramics. Ceram Int 45(8):10154–10162.
Hegenbarth E (1978) The influence of hydrostatic pressure on the phase transitions of some. Ferroelectrics 20(1):79–86.
Bai W et al. (2016) Phase evolution and correlation between tolerance factor and electromechanical properties in BNT-based ternary perovskite compounds with calculated end-member Bi (Me 0.5 Ti 0.5) O 3 (Me = Zn, Mg, Ni, Co). Dalt Trans 45(36):14141–14153.
Liu W, Wang J, Ke X, Li S (2017) Large piezoelectric performance of Sn doped BaTiO3 ceramics deviating from quadruple point. J Alloy Compd 712:1–6.
Zang J, Jo W, Zhang H, Rödel J (2014) Bi1/2Na1/2TiO3–BaTiO3 based thick-film capacitors for high-temperature applications. J Eur Ceram Soc 34(1):37–43.
Puli VS, Pradhan DK, Pérez W, Katiyar RS (2013) Structure, dielectric tunability, thermal stability and diffuse phase transition behavior of lead free BZT–BCT ceramic capacitors. J Phys Chem Solids 74(3):466–475.
Puli VS, Pradhan DK, Riggs BC, Adireddy S, Katiyar RS, Chrisey DB (2014) Synthesis and characterization of lead-free ternary component BST–BCT–BZT ceramic capacitors. J Adv Dielectr 4(02):1450014
Zhang D, Gan Y, Chen T, Yang S, Zhang Y (2015) Phase transition and enhanced electrical properties in 0.725 BiFeO3-0.275 Ba0. 85Ca0. 15Ti0. 9Zr0. 1− xSnxO3 multiferroic ceramics. J Alloy Compd 637:137–142.
Wang J, Zhang X, Zhang J, Li H, Li Z (2012) Dielectric and piezoelectric properties of (1− x) Ba0. 7Sr0. 3TiO3− xBa0. 7Ca0. 3TiO3 perovskites. J Phys Chem Solids 73(7):957–960.
Tripathy N, Singh KC (2022) Probing the dielectric behavior of lead-free BCST ceramic composite: An impedance spectroscopic approach. Mater Today Proc 59:489–495.
Agarwal S, Laishram R, Singh KC (2020) Size-dependent structural and electrical properties of lead-free BCST ceramics prepared from high-energy ball milled nanopowders. Mater Today Commun 25:101400.
Acknowledgements
This work was supported by South valley university of Egypt (83523), National Research Institute of Astronomy and Geophysics (NRIAG) of Egypt (11421).
Author contributions
GMR: methodology, writing–original draft. AAE: investigation, supervision. EMS: software. MAS: writing–review & editing, supervision. All authors reviewed the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rashwan, G.M., Ebnalwaled, A.A., Saad, E.M. et al. Ca/Sn concentration-dependent enhancement of barium titanate ferroelectric performance: a dielectric and microstructural study. J Sol-Gel Sci Technol 109, 707–719 (2024). https://doi.org/10.1007/s10971-023-06306-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06306-4