Abstract
In the present study, curcumin-loaded titania nanofibers were synthesized by the conventional sol-gel method via the electrospinning technique using polyvinyl pyrrolidone and characterized via SEM, FTIR, and XRD. Moreover, their contact angle measurement, degradability, water uptake, and mechanical strength were investigated, besides their antibacterial activity, antioxidant activity, sustained drug release, and cytotoxicity against normal cells and breast cancer cell lines. It can be observed that as the concentration of curcumin increased, the mechanical characteristics of the samples were enhanced. The antibacterial results declared that the increase in curcumin concentration enhanced the inhibitory effect against the different pathogens. Moreover, the viability of cells of the normal cell line, seeded on the samples was in the range from 61.4 to 86.5% which indicated its safety on normal cells. However, the cell viability on the cancerous cell line was found to be 33.8, 19.7, 17.9 and 0% for 0, 1, 3, and 5% loaded TiO2/PVP respectively. This lethal effect of the prepared mats on breast cancer cells with nearly no cytotoxic effect on normal cells, favors the usage of the prepared 5% curcumin-loaded TiO2/PVP mat as anticancerous wound healing bandages for postoperative breast cancer surgeries.
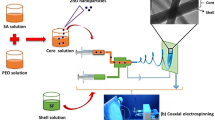
Graphical Abstract

Highlights
-
Novel curcumin loaded titania nanofibers was prepared and characterized.
-
Drug release profile, antioxidant and antimicrobial properties were studied.
-
Cytotoxicity on both normal and cancer cells were evaluated.
-
The produced mats showed a sustained release of curcumin.
-
It showed lethal effect on cancer cells with a nontoxic effect on normal cells.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Breast cancer has the second highest occurrence rate among all types of cancer globally and is the most commonly diagnosed form of cancer [1]. For the majority of solid tumors detected in the early stages, surgical removal of the tumor remains the preferred method of treatment [2]. Despite efforts to completely remove the tumor through surgery, it may not always be successful, and any cancerous cells that remain in the surrounding tissues can lead to cancer coming back in the same area (known as local recurrence) [3]. As a result, there is a need to create new materials that can rapidly halt bleeding and also prevent the reappearance of tumors [2]. One significant challenge in cancer treatment is the lack of specificity of drugs used, as they not only eliminate malignant cells but also affect healthy cells. To minimize the damage to normal cells, it is crucial to maintain an appropriate concentration of the anticancer drug at the site of the surgery.
Nanofibers have the ability to carry anticancer drugs and provide a steady release of the drug at the targeted area. This makes them a valuable tool in minimizing the chances of cancer recurrence in the same area after surgery, as they can be implanted directly into the solid tumor cells for treatment [4]. Researchers are interested in titanium dioxide (TiO2) because it is a cheap material, environmentally friendly, and has very high chemical stability, alongside unique optical and electronic properties [5]. This transition metal oxide occurs naturally in four distinct polymorphs [6]. Nanofibers made of titania have a significantly large active surface area, as well as a favorable distribution of pore sizes making them applicable in various fields, including solar cells, catalytic filters, battery materials, supercapacitors, sensors, environmental, and biomedical uses [7,8,9,10,11,12,13,14]. TiO2 is a safe substance and has been approved by the American Food and Drug Administration (FDA) for its usage in food and medical products [15, 16]. Nano-TiO2 is commonly utilized in medical applications because of its biocompatibility, low toxicity, and flexible manufacturing techniques besides its antibacterial and wound healing properties [17,18,19,20,21]. There are various methods for producing titanium nanofibers, including electrospinning, template, and hydrothermal techniques [22].
The most adaptable approach to creating titanium nanofibers is by the combination of the sol-gel and the electrospinning techniques. This method consists of using a titanium precursor along with a biodegradable polymer like polyvinyl pyrrolidone (PVP) [5, 23, 24]. PVP was chosen as the primary polymer due to its ability to dissolve easily in acetic acid, alcohol, and certain titania precursors [25]. TiO2 is usually used as a biomaterial in its nanoparticles or nanotubular form, no reported studies up to our knowledge used sol-gel electrospun TiO2 as a biomaterial.
When it comes to dealing with cancer cells, careful consideration must be given to the selection of polymer, drug, fabrication technique, and post-modification, as all of these factors are crucial [4]. Recent researches indicate that natural products are a promising role in cancer treatment. Many of the anticancer drugs used in clinical settings are either natural products or derived from them, sourced from various origins including plants and microorganisms [26]. Studies have demonstrated that curcumin, a plant-derived polyphenol obtained from turmeric, can effectively suppress several types of cancerous cells [2]. Curcumin possesses powerful medicinal qualities, including its ability to combat cancer. Specifically, it can trigger apoptosis, or programmed cell death, in cancer cells found in the breast, colon, prostate, and lungs, while leaving healthy cells unharmed. By activating signals that lead to cell death, curcumin can prevent the growth and spread of tumors without any harmful effects on normal cells. This feature nominates it as a promising candidate for inhibiting the progression of cancer [26]. Although curcumin has several benefits, its effectiveness as a therapeutic drug is restricted due to its low water solubility and bioavailability, which pose challenges for drug delivery. To overcome these limitations, researchers have explored alternative methods of administering curcumin. One such method involves incorporating curcumin into nanofibers using electrospinning. These nanofibrous mats have a high surface area-to-volume ratio and porosity, which allows for sustained release of curcumin at the tumor site. This approach reduces the breakdown of curcumin and enhances its accumulation at the tumor site, ultimately improving its therapeutic efficacy [2]. As a result, the use of nanofibers is a significant beneficial approach in minimizing the possibility of cancer recurrence in the surgical area [4]. In the context of breast cancer treatment, the application of electrospun nanofibers loaded with curcumin has shown a notable initial burst of curcumin release, followed by sustained and prolonged release. This suggests that the use of such nanofibers is safe for cancer therapy [27].
In the present study, curcumin was loaded on the as-prepared titania nanofibers via electrospinning technique, to develop a sustained drug release system for localized breast cancer therapy. The resulting nanofibrous mats were analyzed and evaluated for their potential as an anticancer bandage to be used after breast cancer removal surgeries, by testing them on both normal and malignant cells.
2 Materials and methods
2.1 Materials
Titanium (IV) isopropoxide in the form of liquid with a molar mass of 284.26 g/mol and polyvinyl pyrrolidone (PVP) with a molecular weight of 360,000 g/mol (purity of both >99%) are both bought from Sigma-Aldrich. Acetic acid (purity of 96%) and ethanol (purity of 99.8%) are obtained from Edwic, Egypt. Curcumin (CUR, of Mw of 368.38 g/mol) is purchased from Sigma-Aldrich, and was used as an anticancer drug model.
2.2 Solutions preparation
TiO2/PVP solution was prepared according to our previous study [28]. Briefly, calculated grams of PVP were dissolved in 2 ml ethanol to form a 10 wt% PVP solution, and the resulting solution was constantly stirred for 3 h until the formation of a transparent homogenous solution. Then, the calculated amount of titanium tetraisopropoxide (TIP) was dissolved in a solution of 2 ml of acetic acid and ethanol (1:1). The two prepared solutions were mixed and kept on the stirrer at room temperature for 3 h. This sample was nominated as an S sample.
For the CUR-TiO2/PVP solution, different calculated amounts of CUR were dissolved in TiO2/PVP solution and then the same previous steps mentioned above concerning the preparation of the S sample were followed. After stirring, the color of the mixture turned from yellow to dark orange. The obtained samples of 1% of CUR (wt/wt) loaded on TiO2/PVP were termed C1, C3 (3% of CUR loaded on TiO2/PVP), and C5 (5% of CUR loaded on TiO2/PVP).
2.3 Electrospinning
The electrospinning parameters were optimized in our previous study [28]. The prepared solutions of the different samples were poured into a syringe with a metal blunt-ended needle. The positive electrode from the power supply (Glassman High Voltage, (NJ08829)) to the needle of the syringe while the ground electrode was attached to the working platform (collector) and was placed below the tip of the needle. The emerging fibers were obtained from the aluminum foil that cover the collector after adjustment of the electrospinning system at 20 kV voltage, 10 cm distance between the tip of the needle and collector, and a flow rate of 1 ml/h for the collection of the fibers. The electrospinning was performed at room temperature. The samples were labeled as P, PC1, PC3, and PC5.
2.4 Characterizations
The FESEM, Jeol JXA 840, was utilized to study the change in the surface morphology of the electrospun fibers of samples P, PC1, PC3, and PC5. To determine the diameter of the resulting fibers, 50 individual fibers were measured using Image J 1.429 software (NIH, Betheseda, Maryland, USA) [29]. Viscosity measurements of the solutions of P, PC1, PC3, and PC5 were examined at room temperature (25 °C) using a Brookfield viscometer (DV-ІІІ ultra) at 50 RPM using S21 spindle under uniform temperature [30]. The conductivity measurement of various solutions was examined at 25 °C thrice and results were averaged, via a conductivity meter (Model: HC 3010, Trans-instruments). To characterize the chemical structure of electrospun nanofibers of PC1, PC3, PC5, P, CUR alone (pure CUR), and PVP alone (pure PVP) samples were investigated by FTIR for all samples. The FTIR Spectrophotometer (6100-type A, JASCO) was used to perform ATR-FTIR in the 4000 to 400 cm−1 range, with a resolution of 4 cm−1. XRD was used for the physical characterization of electrospun fibers using an X-Ray Diffractometer (CuKα-target with monochromator which operates at 40 kV and 40 mA, Bruker D8, Advance). The hydrophilicity of the electrospun fibrous mats of the samples was determined via contact angle measurement using a horizontal camera perpendicular to the liquid droplet plane, the water droplet was captured after 10 s and analyzed by ImageJ software. The experiment was done for three repeated trials. Then, the mean contact angle and standard deviation were calculated. The electrospinning nanofibrous mats were subjected to tensile tests using a Universal Testing Machine (Lloyd Instruments Ltd LR 10 K) at a rate of 10 mm/min. The specimens’ ends were fixed onto mechanical gripping units with a 2 cm gap length in a vertical position. Each mat was tested five times, and the outcomes were averaged.
2.5 Porosity of the nanofibrous mats
The porosity of the obtained fibers was calculated by obtaing the density from the average of three different speicmens then calculating the porosity by using the following equation [31]
2.6 Antibacterial activity
The antibacterial activity of the nanofibrous mats of the samples P, PC1, PC3, and PC5 was investigated by the disc diffusion method using Gram-positive bacteria such as Staphylococcus aureus (S.aureus) and Gram-negative bacteria such as Escherichia coli (E.coli), Pseudomonas aeruginosa (P.aeruginosa) and Klebsiella pneumonia (K.pneumonia). An extraction was made from the nanofibrous mats with 80% methanol and filtered until ethanol evaporation then a small amount of distilled and sterilized water was added. In this experiment, Mueller Hinton agar media was first filled into the sterilized petri plates and kept overnight at room temperature to ensure that there was no contamination. The different types of bacteria were then grown in nutrient broth for 24 h. Using a sterile cork-borer, circular wells of 6 mm diameter were made in the plates. The wells were loaded with 100 µl of the test substance and the plates were left in the incubator at 37 °C for 24 h. After 24 h, the inhibition zone diameters around the discs were taken using a transparent scale in millimeters. A positive control of an antibiotic and a negative control of distilled sterilized water was also used while performing the experiment [32].
2.7 Antioxidant activity
The ability of the nanofibers to scavenge DPPH was used as an indicator to assess their antioxidant activity [33]. The ability to scavenge radicals was assessed by measuring the capacity to neutralize stable 2,2-diphenyl-2-picrylhydrazylhydrate (DPPH) using a method that was slightly modified from Brand-Williams’s protocol [34]. When an antioxidant compound donates hydrogen to DPPH, it reduces the DPPH, causing a change in color from deep violet to light yellow detected by a UV-visible spectrophotometer at 517 nm. A fresh solution of DPPH (0.1 mM in ethanol) was freshly prepared before taking UV measurements. About 1.8 mg (1 cm × 1 cm) of P, PC1, PC3, and PC5 samples were incubated in the dark in DPPH solution (1 mL) for 0.5, 1, 2, 4, 8, 16, and 24 h at room temperature and the absorbance reading was taken. The experiment was conducted three times. The antioxidant activity of the nanofibers was calculated using the following formula [35].
Where B = absorption of the blank sample at zero time, T = absorption of the test sample for different time intervals [36].
2.8 In vitro drug release
Phosphate-buffered saline (PBS, pH 7.4) with 0.5% Tween-20 was used to investigate the release of curcumin from nanofibrous mats of the samples PC1, PC3, and PC5. This medium was chosen because of the restricted solubility of curcumin in PBS.
All the nanofibrous mats were cut into pieces of equal dimensions (1 cm × 1 cm) and their weights were accurately recorded then each sample was put into a centrifuge tube filled with 10 mL PBS and kept at 37 °C and 120 r/min. 2 ml of the released medium was extracted at regular time intervals for a period of (0.5 h, 1 h, 3 h, 6 h, 24 h, 48 h, 3 days, 7 days and 14 days) for analysis and replaced with fresh PBS. The absorbance of curcumin released per sample was measured using a UV-visible spectrophotometer at a wavelength of 425 nm. All samples were in triplicates and the results were reported as average values. The CUR’s absorbance is converted to concentration according to the calibration curve of CUR in the same buffer. The weight of the released CUR was measured over a period of time, and the percentage of CUR released at each time point was calculated relative to the total amount of CUR loaded.
To determine the amount of curcumin released from the samples, the researchers placed the original drug-loaded fiber mats into vials containing a mixture of ethanol and acetic acid at a ratio of 2:1 v/v. After a few minutes, the fiber mats were completely degraded, indicating that the drug had been released into the solution and measured by a UV spectrophotometer at a wavelength of 425 nm. The concentration of the drug was determined based on a calibration curve. The researchers calculated the total content of the drug in the fiber by taking the average of the three fiber mats. They then back-calculated the actual amount of curcumin released using the same method. The release percentages of Curcumin nanofibers were calculated according to the following equation:
Where t represents a specific immersion time [37].
Several models can be used to depict drug dissolution patterns, where the quantity of drug dissolved from the delivery system is linked to the time function (t). The commonly used and most effective drug release models include the Higuchi model, zero-order model, first-order model, and Korsmeyer-Peppas model. Korsmeyer and his colleagues developed a straightforward relationship that depicted the drug release from a polymeric system [38].
2.9 Swelling Index Study
Swelling is an important property in biodegradable materials for wound healing applications. To determine the percentage of water absorption, the electrospun fibrous mats of P, PC1, PC3, and PC5, samples were cut into equal dimensions (1 cm × 1 cm) specimens and weighted (Wi), then they were immersed in 10 ml PBS in sealed vials at 37 °C. After a predetermined time, 24 h, samples were withdrawn from the vials. The surface water of the mats was adsorbed by filter papers and weighed (Ww). The swelling ratio of the samples is observed according to the following equation [17]:
Where Ww is the wet weight and Wi is the initial weight. The test was done for three repeated trials.
2.10 In vitro degradation
For the weight loss determination (degradation), the electrospun fiber mats of P, PC1, PC3, and PC5 samples were cut into specimens of equal dimensions (1 cm × 1 cm) and weighted (Wi), then they were put in 10 ml PBS in sealed vials at 37 °C. At regular intervals for a period of (1, 3, 7, 14, and 30 days), samples were withdrawn from the vials and they were dried at the time room temperature (Wd).
The weight loss (%) is then calculated according to the following formula [39]:
The test was done for three repeated trials.
2.11 Cytotoxicity assay of different samples
2.11.1 Cell Culture
There are two types of cell lines were used in this experiment: a human normal fibroblast cell line called BJ1 and a human Caucasian breast adenocarcinoma cell line known as MCF-7. Both types of cells were batch cultured for 10 days, under 5% CO2 using a water-jacketed Carbon dioxide incubator. The entire process was conducted in a sterile environment using a laminar flow cabinet biosafety class II level (Baker, SG403INT). The cells were suspended in DMEM-F12 medium, which contained 10,000 µg/ml streptomycin sulfate, 10,000U/ml potassium penicillin, and 25 µg/ml amphotericin B and 1% L-glutamine and were incubated at 37°C with 5% CO2.
2.11.2 Cell Viability Test (Cytotoxicity)
Cell viability test is used to measure the metabolic activity and viability of cells in vitro. This test utilizes a water-soluble tetrazolium salt called MTT, which is yellow in color and selectively interacts with the succinate dehydrogenase enzyme present in the mitochondria of viable cells to form dark blue formazan crystals (water insoluble). This colorimetric technique provides a quantitative measure of cell viability [40]. After the MTT assay, the formazan crystals produced are solubilized by dimethyl sulfoxide (DMSO), to create a colored solution, its intensity depends on the material concentration [33].
Cells (10 × 103) were placed in each well of 96-well plates containing complete growth medium and incubated at 37 °C for 24 h. After 24 h, the medium was removed, and the same amount of fresh medium was added. The cells were then incubated alone (negative control) or with the samples. After 48 h, the medium was removed again, and each well was treated with 40 μL of MTT salt (2.5 μg/ml) and incubated for an additional 4 h at 37 °C with 5% CO2. To halt the reaction and dissolve the crystals that had formed, 200 μL of deionized water containing 10% SDS was introduced to each well and left to incubate overnight at 37 °C [41].
After incubation, the dissolved formazan crystal solution was subjected to absorbance measurements using a multi-well reader (Bio-Rad Laboratories Inc., model 3350) at a wavelength of 595 nm with a reference wavelength of 620 nm [42]. DMSO is used as a negative and its final concentration in the cells was less than 0.2%.
The change in viability (%) was determined according to the following equation:
2.12 Statistical analysis
The reported values represent the mean ± standard deviation. Statistical analysis was carried out using a one-way ANOVA test, which was performed using Excel software. A p-value less than 0.05 was considered statistically significant.
3 Results and discussion
3.1 Fiber morphology
The surface morphology and the mean fiber diameter distribution of the samples P, PC1, PC3, and PC5 nanofibers were characterized using SEM and are presented in Fig. 1. All the nanofibers showed smooth nanoscale fibrous structure. Loaded curcumin showed smooth, beadless nanofibers, but the incorporation of curcumin resulted in a significant increase in fiber diameter [43]. The mean fiber diameters of the samples were 226.3 ± 73.5 nm, 501.2 ± 153.3 nm, 550.3 ± 137.5 nm, and 556.4 ± 149.5 nm respectively. The mean mat porosity was found to be 96.41, 96,58, 93.08, 96.09% of the samples P, PC1, PC3, and PC5 nanofibers respectively. The PC1 & PC3 samples exhibited smooth, beadless nanofibers and were free from any aggregates confirming the inclusion of loaded curcumin in the nanofibers, while PC5 nanofibers showed the presence of multiple beads along the fibers since the formation of beads in the fibers was increased with by CUR increase. This may be due to the increase in the viscosity as reported later [44, 45]. These findings are consistent with the results reported by Shababdoust et al. in their study of adding curcumin to polyurethanes [46]. The results obtained in this study are also consistent with the findings of Thangaraju et al. in their research on the impact of curcumin on Poly L-Lactide [47]. This increase was attributed to the increased viscosity due to the interaction between curcumin and TiO2/PVP.
3.2 Rheological measurements
The viscosity of the electrospinning solution plays an important role in the morphology and electrospinnability of the nanofibers. Insufficient chain entanglement between polymeric chains can result in bead formation due to low viscosity, while blockage at the capillary tip can occur due to excessively high viscosity. According to the results recorded in Table 1, the viscosity increased with the addition of curcumin. This result is inconsistent with the results obtained by Mohebian et al., they also found an increase in viscosity resulting from the CUR addition [48]. This viscosity increment might be due to the interaction between TiO2/PVP and curcumin as observed from XRD and FTIR, thus causing the increase of the fiber diameters of Cur loaded on TiO2.
3.3 Conductivity measurements
The conductivity of the solution containing curcumin is higher than that of TiO2/PVP which indicates that; the addition of CUR to the TiO2/PVP solution increases its conductivity [43]. As illustrated in Table 2. These results match with the results obtained by Caglayan and Basal who noticed that the increase in conductivity of pure PCL solution occurs with the addition of curcumin and silk fibroin [43].
3.4 X-ray diffraction
The crystalline nature of the uncalcinated electrospun nanofibers was studied by X-ray diffraction (XRD) measurement. The XRD patterns of the electrospun samples of P, PC1, PC3 & PC5 samples are shown in Fig. 2. The XRD pattern of the sample P shows an amorphous nature since sample P represents the uncalcinated nanofibers formed from TiO2/PVP. There is no peak observed for PVP, indicating that the addition of PVP to titanium peroxide reduces the crystalline nature of PVP and increases its amorphous nature. Moreover, there is no peak for TiO2, indicating good mixing between PVP and TiO2 in the samples [49,50,51]. These findings are consistent with Someswararao explanation that the XRD patterns of TiO2/PVP and TiO2 nanofibrous mats before calcination did not show any diffraction peaks, indicating the TiO2/PVP mat has an amorphous nature [50]. The XRD patterns of PC1, PC3, and PC5 showed a significant absence of the crystalline peaks of curcumin, indicating that CUR was not present in the crystalline form in the fibrous mats and was effectively incorporated within the PVP. Earlier studies have reported that CUR loses its crystallinity by incorporating an amorphous polymer, which rationalizes the absence of CUR peaks in these samples. These results are consistent with the findings of Mahmud et al, who reported the absence of CUR peaks in the XRD patterns of PVA/CUR [51].
3.5 FTIR analysis
FTIR spectroscopy is performed to characterize the functional groups present in the fibers by analyzing the energy levels and stretching capabilities of the chemical bonds. FTIR spectrum of the samples P, PC1, PC3, PC5, CUR alone (pure CUR) & PVP alone (pure PVP) are represented in Fig. 3. Four distinct peaks are observed in the spectrum of pure CUR. The peak at 1627 cm−1 was assigned to the stretching of the C = O group, which was shifted to a lower wavenumber than the typical C = O stretching range (1680–1750 cm−1) due to the presence of conjugation around the carbonyl group. Additionally, the phenolic C-O stretching is observed at 1428 cm−1 [52]. The peak at 1455 cm−1 and the peak at 1377 cm−1 could be assigned to the bending vibration of CH2 and CH3 groups respectively.
The FTIR spectra of PVP show a band at 1664 cm−1, which is associated with the pyrrolidone C = O group. The band observed at 1647cm−1 corresponds to the C = O stretching of the PVP. Additionally, the peak at 2945 cm−1 reveals the asymmetric stretching of the CH2 group in C-H bonds. The peak at 1286 cm−1 is related to the C-N stretching vibration [53]. The FTIR spectra of TiO2 displayed several vibration peaks. The peak at 2955 cm−1 is assigned to the stretching vibration of the C-H group, while the C = O vibration stretching mode is observed at 1654 cm−1. The bending vibration of the CH2 group is indicated by the peak at 1462 cm−1, and the stretching vibration of the C-N group of the polymer is assigned to 1291 cm−1. Additionally, the peak at 648 cm−1 is related to the characteristic Ti-O-Ti bonds [50]. The peaks at 1019 and 1020 cm−1 correspond to the C-O stretching vibration of isopropoxide groups attached to Ti atoms, while the peak at 1440 cm−1 can be assigned to the symmetrical vibration of the CO2 functional groups bound to the Ti atoms. The formation of Ti-O-Ti bonds can be identified by the existence of broadband at wave numbers below 800 cm−1. At the low wavenumber region (800–400 cm−1) we can see the continuous broad spectrum with a peak at 575 cm−1 which is attributed to Ti-O-Ti stretching vibration [54]. From the FTIR spectra of the samples PC1, PC3 & PC5, we can detect the same characteristic peaks which are 2955 cm−1 is assigned to the stretching vibration of the C-H group, while the C = O vibration stretching mode is observed at 1654 cm−1. The bending vibration of the CH2 group is indicated by the peak at 1462 cm−1, and the stretching vibration of the C-N group of the polymer is assigned to 1291 cm−1. There are nearly no changes or appearance of any peaks characteristic to the loading of curcumin. However, there is an increment in some peaks’ intensity with the addition of curcumin. This may be referred to the inclusion of curcumin inside the electrospun nanofibers.
3.6 Mechanical measurements
The stress-strain curves of samples P, PC1, PC3, and PC5 are displayed in Fig. 4, and the related parameters are represented in Table 3. It is shown from the results that the mechanical properties of sample P were enhanced by the addition of the curcumin for the samples PC1, PC3, and PC5. This enhancement could be assigned to the increase in fiber diameter resulting from the interaction between curcumin, and TiO2/PVP that increased the density, which subsequently led to increased mechanical strength of the material. Furthermore, the homogeneous distribution of the CUR along the nanofibers played a role in enhancing its mechanical strength [55] The percentage elongation at break of P, PC1, PC3, and PC5 nanofibrous mats were found to be 5.75, 5.89, 6.01, and 6.16% respectively, indicating that the presence of CUR contributes to further elongation of the fiber mats. PC5 shows the highest tensile modulus, while P showed the lowest tensile strength. Young’s modulus was calculated using the slope of the stress-strain plot’s linear portion. As presented in Table 3, Young’s modulus of the samples P, PC1, PC3, and PC5 nanofibrous mats are found to be 6.67, 11.99, 19.22, and 25.21 MPa respectively. The maximum tensile strength of the samples P, PC1, PC3, and PC5 nanofibrous mats was found to be 0.161, 0.235, 0.368, and 0.502 MPa respectively. These findings are in match with those obtained by Niranjan et al. who found that incorporating TiO2-Cur into the polymer increased its tensile strength [56].
3.7 Contact Angle Measurements
The contact angle is a valuable method for investigating the impact of additives on surface properties. It is commonly used to determine the hydrophobic effect of water or aqueous solutions on surfaces. Surface wettability plays an important role in the biocompatibility and biodegradation of biomaterials [57]. Surface wettability was categorized into three classes which are: (a) hydrophilic specimen, which has a contact angle ranging from 0–30°, (b) moderately hydrophilic specimen, which has a contact angle between 30–90° and (c) hydrophobic specimen, which has a contact angle greater than 90° [30]. The results of the contact angle measured after 10 s for samples P, PC1, PC3 &PC5 are shown in Table 4. The contact angle of the sample P increases after the incorporation of CUR which indicates increased hydrophobicity due to the hydrophobic nature of CUR.
3.8 Swelling study
The swelling index (water uptake) at the test environment (PBS) at 37 °C after the predetermined time of 24 h for samples P, PC1, PC3, and PC5 is shown in Fig. 5a. The swelling index of the samples decreases by increasing CUR with statistical significance between all the samples. This can be attributed to the hydrophobic nature of CUR which prevents the water inclusion leading to less water attachment to the surface of the fibers by the increase of CUR.
3.9 In-vitro biodegradation measurement
In vitro, biodegradation of electrospun samples P, PC1, PC3, and PC5 are examined by weight loss measurements after 1, 3, 7, 14, and 30 days in PBS (pH 7.4). As illustrated in Fig. 5b, the degradation rate of the samples shows a gradual increase with time intervals, besides, drug-loaded nanofibers showing higher degradation. This may be due to the CUR’s elution, which creates more sites for water to interact, increasing the degradation of the samples. Degradation of the material is beneficial in forming a new extracellular matrix (ECM) and creating space for cell proliferation and differentiation [55, 56].
3.10 In-vitro drug release
The drug release from nanofibers is influenced by different factors such as the extent of degradation, swelling, and the porosity of the mat’s surface [55]. To determine the percentage of CUR released from the samples, the absorbance of the testing samples was measured and compared to a calibration curve. The initial curcumin concentration resulted from dissolving (2 × 1 cm2) fibrous mats was found to be 0.00023 ± 0.00012, 0.00055 ± 0.00019 and 0.00130 ± 0.000242 mg/ml for the samples of PC1, PC3, and PC5 respectively. Figure 6a, b describes the results of the cumulative drug release mass (mg) and percentage (%) of CUR released from the samples of PC1, PC3, and PC5 electrospun nanofibers. The behavior of CUR release profiles is similar for all three different electrospun samples and shows 2 stages of release. Through stage (І), there is an initial burst release (fast release) within 48 h followed by decelerating release profile at stage (П). The cumulative drug release of CUR during stage (І) reaches about 66.88% ± 0.003, 61.58% ± 0.003 and 72.80% ± 0.022 for the samples PC1, PC3, and PC5 respectively. The burst release was thought to be due to CUR being present on or near the nanofibrous surface. The second stage showed a gradual release of curcumin, with cumulative drug release reaching about 84.43% ± 0.010, 72.0% ± 0.014, and 80.34% ± 0.027 for PC1, PC3, and PC5 respectively. At the end of these stages, the release rate of CUR is gradually decelerating, ultimately reaching a plateau for the various samples.
The cumulative drug release of CUR at the last period of immersion (336 h) reaches 99.0% ± 0.007, 91.1% ± 0.008, and 90.6 % ± 0.015 for the samples PC1, PC3, and PC5 respectively. The initial release of loaded CUR determined in the present work is slower than that by other authors [2, 43]. Typically, a burst release of drugs is not preferred for controlled drug release systems. However, in cases of severe infections or immediately after surgery, a burst release of the drug at the wound site may offer advantages [43]. The Korsemeyer-Peppas model was used to determine the rate and mechanism of drug release, as shown in Fig. 6c. Both PC1, PC3, and PC5 exhibited good linear fitting with the Korsemeyer-Peppas model [58], as can be observed from high R2 values (0.9648, 0.9674 and 0.9831) of a drug release from PC1, PC3, and PC5.
3.11 Antibacterial activity
The antibacterial activity of the samples P, PC1, PC3 & PC5 nanofibers against different Gram-positive bacteria such as Staphylococcus aureus (S.aureus), and Gram-negative bacteria such as Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), Klebsiella pneumonia (K. pneumonia) used in this study are represented in Fig. 7 and Table 5. The TiO2/PVP results showed an antibacterial effect due to the antibacterial effect of titanium dioxide [20, 59] However, the results showed that the inhibitory effect of TiO2/PVP with CUR (samples PC1, PC3 & PC5) are higher than that of sample the P (TiO2/PVP only), where the inhibition zone of TiO2/PVP with CUR increased with increasing the concentration of CUR [60, 61]. The mechanism of CUR-bacterial interaction is not completely understood but as reported CUR cause apoptosis like effect to E. Coli beside causing its membrane damage [62] Also, curcumin was found to effectively hinder the virulence factors of Pseudomonas aeruginosa, including the formation of biofilm, synthesis of pyocyanin, activity of elastase/protease, and production of acyl homoserine lactone [63]. It is widely believed that curcumin’s inhibition of the assembly dynamics of bacterial protein and membrane is a key mechanism by which it hampers S. auerus cell proliferation [60].
This result is harmonized with those discussed by Nagalakshmi [32]. Tajbakhsh has also pointed out that curcumin, which is the primary yellow bioactive compound found in turmeric powder, has demonstrated various biological effects, including antimicrobial activity [64]. Furthermore, this result is in agreement with the investigation demonstrated by Teow and also by Zheng [60, 61]. Teow observed that CUR has high antibacterial effects when it is combined with other antibacterial agents. Also, Zheng [61] has reported that CUR exhibited strong biological effectivity and broad-spectrum antibacterial activity against both Gram-positive and Gram-negative bacteria. CUR can achieve this by targeting various cellular structures of bacteria, such as the cell wall, cell membrane, protein, and DNA, thereby inhibiting bacterial growth.
3.12 Antioxidant activity
Antioxidants are substances that neutralize reactive oxygen species (ROS) and their actions. The effectiveness of antioxidants depends on various factors such as the polarity and the type of extracting solvent, the purity of active compounds, and the isolation procedures. Additionally, the used assays and the substrate used play a role in determining the antioxidant activity of a substance [65, 66]. Following any injury, the formation of reactive oxygen species can impede the healing activity by inducing tissue damage in the wound area. These ROS can have a particularly negative impact on the fibroblast cell’s adhesion and proliferation. Therefore, it is necessary to eliminate these species from the wound site to avoid prolonged wound healing times [33]. Figure 8, shows the time-dependent change in the antioxidant activity of samples P, PC1, PC3 & PC5. It was shown that the antioxidant activity was increased (remaining DPPH% decreased) with increasing both curcumin percent and incubation time. Therefore, all samples exhibited a stable antioxidant capacity after 8 h, where the inhibition % was almost equal after 16 and 24 h, indicating the absence of hydrogen atoms that can be donated for quenching DPPH free radicals due to CUR total release [67, 68]. The interaction between the antioxidant and DPPH is dependent on the chemical structure of the antioxidant. Hence, the ability of a material to scavenge free radicals and reduce DPPH which is considered an indicator of its antioxidant activity. It is based on its capacity to donate an active hydrogen atom [69] Blank TiO2/PVP nanofibers presented small antioxidant activity (nearly 10% inhibition only) after 24 h. On the other hand, the curcumin-loaded samples showed high antioxidant activity. The results indicated that curcumin kept its antioxidant properties after the encapsulation process indicating that encapsulation was an effective way to improve the antioxidant capacity of curcumin [70]. These results match with the results obtained by Gulsun and his coworkers who noticed that CUR resulted in more than ninety percent antioxidant activity even at high DPPH concentrations [33]. Similarly, the results of this study demonstrate the high radical scavenging capabilities of the samples, even with small amounts of CUR. These findings suggest that wound dressings made from these nanofibers could help prevent inflammation in the wound area by eliminating ROS and lowering inflammatory factors, thereby accelerating the healing process.
3.13 In vitro cytotoxicity effect on BJ1 cells and MCF-7 cells
The in vitro cytotoxicity of the samples P, PC1, PC3 &PC5 nanofibrous mats against BJ1 cells (normal skin fibroblast) by MTT assay. The results are shown in Fig. 9a. Sample P shows high viability, but it is significantly less than the cells cultured on the control well which may be assigned to the hydrophobicity of the P sample. However, it is noted that cells significantly proliferated in PC1 and PC3 samples as compared with the P sample. On the other hand, more than 70% of the cells remained viable on PC5 nanofiber mats. It was found that the viability of cells seeded on P, PC1, and PC3 mats are 61.4%, 66.8%, and 71.7% respectively. Meanwhile, the viability of cells on the PC5 mat is found to be 86.5%. There is a significant difference between all the samples (p < 0.005). Overall, the results indicate that the use of CUR could stimulate cell proliferation, suggesting low cytotoxicity [33, 71].
To verify the pharmacological efficacy of the released CUR, the cytotoxicity of the nanofibers loaded with different CUR concentrations against MCF-7 cell proliferation is evaluated by MTT assay. The in vitro cytotoxicity of the various samples including P, PC1, PC3, and PC5 nanofibers mats were represented. As shown in Fig. 9b, the study found that cell survivability was reduced when exposed to the nanofibers loaded with CUR. The viability of cells is 19.7%, 17.9%, and 0%, indicating that all cells were dead for the samples PC1, PC3, and PC5 mats respectively. While the viability of cells is about 33.8% for the blank P sample. These results confirmed the improved anti-proliferative effects of CUR-loaded nanofibers against the MCF-7 breast cancer cell line [2]. There is a significant difference between all the samples (p < 0.005). This result is harmonized with those discussed by Sridhar et al. and also by Mohebian et al. as they found that curcumin addition reduced the MCF-7 cell viability and increased its apoptosis induction [48, 72, 73].
4 Conclusions
In this study, novel curcumin loaded as prepared titania nanofibers were produced and characterized for their physical and biological properties. The nanofibers exhibited improved mechanical properties, antioxidant activity, biodegradability, swelling, and antibacterial activity with increased curcumin concentration. The nanofibers also demonstrated no significant cytotoxicity to normal cells but were found to be lethal to breast cancer cells. These findings suggest that the prepared nanofibers could be a promising option for wound-healing bandages after breast cancer surgeries to prevent cancer metastasis.
Data availability
Data is available upon request.
References
Rizvi FH, Khan MK, Almas T, et al (2020) Early Postoperative Outcomes of Breast Cancer Surgery in a Developing Country. Cureus. https://doi.org/10.7759/cureus.9941
Chen K, Pan H, Yan Z et al. (2021) A novel alginate/gelatin sponge combined with curcumin-loaded electrospun fibers for postoperative rapid hemostasis and prevention of tumor recurrence. Int J Biol Macromol 182:1339–1350. https://doi.org/10.1016/j.ijbiomac.2021.05.074
Darbasizadeh B, Mortazavi SA, Kobarfard F et al. (2021) Electrospun Doxorubicin-loaded PEO/PCL core/sheath nanofibers for chemopreventive action against breast cancer cells. J Drug Deliv Sci Technol 64:102576. https://doi.org/10.1016/j.jddst.2021.102576
Abid S, Hussain T, Raza ZA, Nazir A (2019) Current applications of electrospun polymeric nanofibers in cancer therapy. Mater Sci Eng C 97:966–977. https://doi.org/10.1016/j.msec.2018.12.105
Tang ZS, Bolong N, Saad I et al. (2016) Effect of polyvinylpyrrolidone and fabrication parameters on electrospun titanium oxide nanofibres’ diameter. J Teknol 78:19–24. https://doi.org/10.11113/jt.v78.10047
Gupta SM, Tripathi M (2011) A review of TiO2 nanoparticles. Chin Sci Bull 56:1639–1657
Bai J, Zhou B (2014) Titanium dioxide nanomaterials for sensor applications. Chem Rev 114:10131–10176
Bertuna A, Comini E, Poli N, et al (2016) Titanium Dioxide Nanostructures Chemical Sensor. In: Procedia Engineering. Elsevier Ltd, pp 313–316
Dai Y, Formo E, Li H et al. (2016) Surface-functionalized electrospun titania nanofibers for the scavenging and recycling of precious metal ions. ChemSusChem 9:2912–2916. https://doi.org/10.1002/cssc.201600787
Lee S, Eom W, Park H, Han TH (2017) High-temperature stable anatase titanium oxide nanofibers for lithium-ion battery anodes. ACS Appl Mater Interfaces 9:25332–25338. https://doi.org/10.1021/acsami.7b06631
Da Silva EP, Rubira AF, Ferreira OP et al. (2019) In situ growth of manganese oxide nanosheets over titanium dioxide nanofibers and their performance as active material for supercapacitor. J Colloid Interface Sci 555:373–382. https://doi.org/10.1016/j.jcis.2019.07.064
Mustafa MN, Shafie S, Wahid MH, Sulaiman Y (2019) Light scattering effect of polyvinyl-alcohol/titanium dioxide nanofibers in the dye-sensitized solar cell. Sci Rep. 9:1–8. https://doi.org/10.1038/s41598-019-50292-z
Khosravi M, Maddah AS, Mehrdadi N, et al (2020) Synthesis of TiO2/ZnO electrospun nanofibers coated-sewage sludge carbon for adsorption of Ni(II), Cu(II), and COD from aqueous solutions and industrial wastewaters. J Dispers Sci Technol. https://doi.org/10.1080/01932691.2019.1711111
Jafari S, Mahyad B, Hashemzadeh H, et al (2020) Biomedical Applications of TiO 2 Nanostructures: Recent Advances. https://doi.org/10.2147/IJN.S249441
Bonetta S, Bonetta S, Motta F et al. (2013) Photocatalytic bacterial inactivation by TiO2-coated surfaces. AMB Express 3:1–8. https://doi.org/10.1186/2191-0855-3-59
Mccullagh C, Robertson JMC, Bahnemann DW, Robertson PKJ (2007) The application of TiO2 photocatalysis for disinfection of water contaminated with pathogenic micro-organisms: A review. Res Chem Intermed 33:359–375. https://doi.org/10.1163/156856707779238775
Deyab NM, Ekram B, Badr KR et al. (2022) Antiviral electrospun polyamide three-layered mask filter containing metal oxide nanoparticles and black seed oil. ACS Omega 7:44438–44447. https://doi.org/10.1021/ACSOMEGA.2C06611/ASSET/IMAGES/LARGE/AO2C06611_0008.JPEG
Akhtar S, Shahzad K, Mushtaq S, et al. (2019) Antibacterial and antiviral potential of colloidal Titanium dioxide (TiO2) nanoparticles suitable for biological applications. Mater Res Express 6. https://doi.org/10.1088/2053-1591/ab3b27
Grassian VH, O’Shaughnessy PT, Adamcakova-Dodd A et al. (2007) Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ Health Perspect 115:397. https://doi.org/10.1289/EHP.9469
Azizi-Lalabadi M, Ehsani A, Divband B, Alizadeh-Sani M (2019) Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci Rep. 9:1–10. https://doi.org/10.1038/s41598-019-54025-0
GA Seisenbaeva K Fromell VV Vinogradov et al. (2017) Dispersion of TiO2 nanoparticles improves burn wound healing and tissue regeneration through specific interaction with blood serum proteins. Sci Rep 71:1–11. https://doi.org/10.1038/s41598-017-15792-w
Altaf AA, Ahmed M, Hamayun M et al. (2020) Titania nano-fibers: a review on synthesis and utilities. Inorg Chim Acta 501:119268
Nuansing W, Ninmuang S, Jarernboon W et al. (2006) Structural characterization and morphology of electrospun TiO2 nanofibers. Mater Sci Eng B Solid State Mater Adv Technol 131:147–155. https://doi.org/10.1016/j.mseb.2006.04.030
Albetran H, Dong Y, Low IM (2015) Characterization and optimization of electrospun TiO 2 /PVP nanofibers using Taguchi design of experiment method. J Asian Ceram Soc 3:292–300. https://doi.org/10.1016/j.jascer.2015.05.001
Park JY, Kim SS (2009) Effects of processing parameters on the synthesis of TiO2 nanofibers by electrospinning. Met Mater Int 15:95–99. https://doi.org/10.1007/s12540-009-0095-7
Pal K, Roy S, Parida PK et al. (2019) Folic acid conjugated curcumin loaded biopolymeric gum acacia microsphere for triple negative breast cancer therapy in invitro and invivo model. Mater Sci Eng C 95:204–216. https://doi.org/10.1016/j.msec.2018.10.071
Mateti T, Aswath S, Vatti AK et al. (2021) A review on allopathic and herbal nanofibrous drug delivery vehicles for cancer treatments. Biotechnol Rep. 31:e00663. https://doi.org/10.1016/j.btre.2021.e00663
Optimization of electro spinning parameters and cytotoxicity evaluation of titanium dioxide nanofibers to be used as a biomaterial (2022). Middle East J Appl Sci. https://doi.org/10.36632/MEJAS/2022.12.4.32
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to imageJ: 25 years of image analysis. Nat Methods 9:671. https://doi.org/10.1038/NMETH.2089
Ekram B, Abd El-Hady BM, El-Kady AM, et al. (2019) Enhancing the stability, hydrophilicity, mechanical and biological properties of electrospun polycaprolactone in formic acid/acetic acid solvent system. Fibers Polymers 20 https://doi.org/10.1007/s12221-019-8795-1
Aghayari S, Aghayari S (2022) The Porosity of Nanofiber Layers. Biocomposites - Recent Advances. https://doi.org/10.5772/INTECHOPEN.109104
Nagalakshmi M, Karthikeyan C, Anusuya N et al. (2017) Synthesis of TiO2 nanofiber for photocatalytic and antibacterial applications. J Mater Sci: Mater Electron 28:15915–15920. https://doi.org/10.1007/s10854-017-7487-0
Gulsun T, Inal M, Akdag Y et al. (2022) The development and characterization of electrospun gelatin nanofibers containing indomethacin and curcumin for accelerated wound healing. J Drug Deliv Sci Technol 67:103000. https://doi.org/10.1016/j.jddst.2021.103000
Brand-Williams W, Cuvelier M.E, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 28:25–30
Abdel-Hady BM, Ekram B, Müller WEG et al. (2023) Ascorbyl palmitate–PCL fiber mats loaded with strontium polyphosphate nanoparticles for guided bone regeneration. Polymer Bull 1–20. https://doi.org/10.1007/S00289-023-04868-5
Eldurini S, Abd El-Hady BM, Shafaa MW et al. (2021) A multicompartment vascular implant of electrospun wintergreen oil/ polycaprolactone fibers coated with poly(ethylene oxide). Biomed J 44:589–597. https://doi.org/10.1016/j.bj.2020.04.008
HT Bui OH Chung JS Park (2014) Fabrication of electrospun antibacterial curcumin-ioaded zein nanofibers. Polymer (Korea) 38:744–751. https://doi.org/10.7317/pk.2014.38.6.744.
Singh M, Pilani S (2021) Review: in vitro drug release characterization models. Int J Pharm Stud Res 2:77–84
Kandil H, Ekram B, Abo-Zeid MAM (2022) Cytocompatibility of MG-63 osteosarcoma cells on chitosan/hydroxyapatite/lignin hybrid composite scaffold in vitro. Biomed Mater 18:015002. https://doi.org/10.1088/1748-605X/AC9F92
Ekram B, Abd El-Hady BM, El-Kady AM, et al. (2021) Enhanced mesenchymal stem cells growth on antibacterial microgrooved electrospun zinc chloride/polycaprolactone conduits for peripheral nerve regeneration. J Bioact Compat Polym. https://doi.org/10.1177/0883911520988305
Thabrew MI, Hughes RD, Mcfarlane IG (1997) Screening of hepatoprotective plant components using a HepG2 cell cytotoxicity assay. J Pharm Pharmacol 49:1132–1135. https://doi.org/10.1111/j.2042-7158.1997.tb06055.x
Bassyouni FA, Abu-Baker SM, Mahmoud K et al. (2014) Synthesis and biological evaluation of some new triazolo[1,5-a]quinoline derivatives as anticancer and antimicrobial agents. RSC Adv 4:24131–24141. https://doi.org/10.1039/c3ra46961a
Caglayan B, Basal G (2020) Electrospun polycaprolactone/silk fibron nanofibers loaded with curcumin for wound dressing applications. Dig J Nanomater Biostruct 15:1165–1173
Kulkarni AS, Gurav DD, Khan AA, Shinde VS (2020) Curcumin loaded nanofibrous mats for wound healing application. Colloids Surf B Biointerfaces 189:1–10. https://doi.org/10.1016/j.colsurfb.2020.110885
Rahma, Munir A, Khairurrijal MM et al. (2016) Intermolecular interactions and the release pattern of electrospun curcumin-polyvinyl(pyrrolidone) fiber. Biol Pharm Bull 39:163–173. https://doi.org/10.1248/bpb.b15-00391
Shababdoust A, Ehsani M, Shokrollahi P, Zandi M (2018) Fabrication of curcumin-loaded electrospun nanofiberous polyurethanes with anti-bacterial activity. Prog Biomater 7:23. https://doi.org/10.1007/S40204-017-0079-5
Thangaraju E, Srinivasan NT, Kumar R et al. (2012) Fabrication of electrospun Poly L-lactide and Curcumin loaded Poly L-lactide nanofibers for drug delivery. Fibers Polym 13:823–830. https://doi.org/10.1007/S12221-012-0823-3
Mohebian Z, Babazadeh M, Zarghami N, Mousazadeh H (2021) Anticancer efficiency of curcumin-loaded mesoporous silica nanoparticles/nanofiber composites for potential postsurgical breast cancer treatment. J Drug Deliv Sci Technol 61:102170. https://doi.org/10.1016/J.JDDST.2020.102170
Sharma A, Pathak D, Patil DS et al. (2021) Electrospun PVP/TiO2 nanofibers for filtration and possible protection from various viruses like COVID-19. Technol (Basel) 9:89. https://doi.org/10.3390/technologies9040089
Someswararao MV, Dubey RS, Subbarao PSV, Singh S (2018) Electrospinning process parameters dependent investigation of TiO2 nanofibers. Results Phys 11:223–231. https://doi.org/10.1016/j.rinp.2018.08.054
Mahmud MM, Zaman S, Perveen A et al. (2020) Controlled release of curcumin from electrospun fiber mats with antibacterial activity. J Drug Deliv Sci Technol 55:101386. https://doi.org/10.1016/j.jddst.2019.101386
Wang C, Ma C, Wu Z et al. (2015) Enhanced bioavailability and anticancer effect of curcumin-loaded electrospun nanofiber: In Vitro and In Vivo Study. Nanoscale Res Lett 10:1–10. https://doi.org/10.1186/s11671-015-1146-2
Asmatulu R, Khan WS (2019) Characterization of electrospun nanofibers. In: Synthesis and Applications of Electrospun Nanofibers. pp 257–281
Mirmohammad Sadeghi S, Vaezi M, Kazemzadeh A, Jamjah R (2018) Morphology enhancement of TiO2/PVP composite nanofibers based on solution viscosity and processing parameters of electrospinning method. J Appl Polym Sci 135:1–11. https://doi.org/10.1002/app.46337
Prakash J, Venkataprasanna KS, Bharath G et al. (2021) In-vitro evaluation of electrospun cellulose acetate nanofiber containing Graphene oxide/TiO2/Curcumin for wound healing application. Colloids Surf A Physicochem Eng Asp 627:127166. https://doi.org/10.1016/j.colsurfa.2021.127166
Niranjan R, Kaushik M, Selvi RT et al. (2019) PVA/SA/TiO2-CUR patch for enhanced wound healing application: In vitro and in vivo analysis. Int J Biol Macromol 138:704–717. https://doi.org/10.1016/J.IJBIOMAC.2019.07.125
Serati-Nouri H, Mahmoudnezhad A, Bayrami M et al. (2021) Sustained delivery efficiency of curcumin through ZSM-5 nanozeolites/electrospun nanofibers for counteracting senescence of human adipose-derived stem cells. J Drug Deliv Sci Technol 66:102902. https://doi.org/10.1016/j.jddst.2021.102902
Dash S, Murthy PN, Nath L, Chowdhury P (2010) Kinetic modeling on drug release from controlled drug delivery systems. Acta Poloniae Pharmaceutica - Drug Res 67:217–223
Mokhtari Aghdami R, Mousavi SR, Estaji S et al. (2022) Evaluating the mechanical, thermal, and antibacterial properties of poly (lactic acid)/silicone rubber blends reinforced with (3-aminopropyl) triethoxysilane-functionalized titanium dioxide nanoparticles. Polym Compos 43:4165–4178. https://doi.org/10.1002/PC.26679
Teow SY, Liew K, Ali SA et al. (2016) Antibacterial action of curcumin against staphylococcus aureus: a brief review. J Trop Med 2016:10. https://doi.org/10.1155/2016/2853045
Zheng D, Huang C, Huang H, et al. (2020) Antibacterial mechanism of curcumin: a review. Chem Biodivers 17. https://doi.org/10.1002/cbdv.202000171
Yun DG, Lee DG (2016) Antibacterial activity of curcumin via apoptosis-like response in Escherichia coli. Appl Microbiol Biotechnol 100:5505–5514. https://doi.org/10.1007/S00253-016-7415-X/METRICS
Rudrappa T, Bais HP (2008) Curcumin, a known phenolic from Curcuma longa, attenuates the virulence of Pseudomonas aeruginosa PAO1 in whole plant and animal pathogenicity models. J Agric Food Chem 56:1955–1962. https://doi.org/10.1021/JF072591J/SUPPL_FILE/JF072591J-FILE004.PDF
Tajbakhsh S, Mohammadi K, Deilami I et al. (2008) Antibacterial activity of indium curcumin and indium diacetylcurcumin. Afr J Biotechnol 7:3832–3835. https://doi.org/10.4314/ajb.v7i21.59452
Meyer AS, Heinonen M, Frankel EN (1998) Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin, and ellagic acid on human LDL oxidation. Food Chem 61:71–75. https://doi.org/10.1016/S0308-8146(97)00100-3
Benites RSR, Formagio ASN, Argandoña EJS et al. (2015) Contents of constituents and antioxidant activity of seed and pulp extracts of Annona coriacea and Annona sylvatica. Braz J Biol 75:685–691. https://doi.org/10.1590/1519-6984.21313
Naik GH, Priyadarsini KI, Satav JG et al. (2003) Comparative antioxidant activity of individual herbal components used in ayurvedic medicine. Phytochemistry 63:97–104. https://doi.org/10.1016/S0031-9422(02)00754-9
Banerjee S, Saikia JP, Kumar A, Konwar BK (2010) Antioxidant activity and haemolysis prevention efficiency of polyaniline nanofibers. Nanotechnology 21:045101. https://doi.org/10.1088/0957-4484/21/4/045101
Gizdavic-Nikolaidis M, Travas-Sejdic J, Bowmaker GA et al. (2004) Conducting polymers as free radical scavengers. Synth Met 140:225–232. https://doi.org/10.1016/S0379-6779(03)00372-2
Meng R, Wu Z, Xie QT et al. (2021) Preparation and characterization of zein/carboxymethyl dextrin nanoparticles to encapsulate curcumin: Physicochemical stability, antioxidant activity and controlled release properties. Food Chem 340:127893. https://doi.org/10.1016/j.foodchem.2020.127893
Bui HT, Chung OH, Dela Cruz J, Park JS (2014) Fabrication and characterization of electrospun curcumin-loaded polycaprolactone-polyethylene glycol nanofibers for enhanced wound healing. Macromol Res 22:1288–1296. https://doi.org/10.1007/s13233-014-2179-6
Sridhar R, Ravanan S, Venugopal JR et al. (2014) Curcumin- and natural extract-loaded nanofibres for potential treatment of lung and breast cancer: in vitro efficacy evaluation. J Biomater Sci Polym Ed 25:985–998. https://doi.org/10.1080/09205063.2014.917039
Mitra S, Mateti T, Ramakrishna S, Laha A (2022) A review on curcumin-loaded electrospun nanofibers and their application in modern medicine. JOM 74(1989):3392–3407. https://doi.org/10.1007/S11837-022-05180-9
Acknowledgements
The Authors would like to thank the National Research Centre and Al-Azhar University for providing facilities for this study.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
SS, BE and AO have contributed in the experimental work and the writing of the manuscript. AE has supervised the study and revised the manuscript. AG did the antioxidant study and wrote it, BA has supervised the study, designed the material and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All cell culture experimental protocols were in accordance with the ethical guidelines of the ethical committee of National Research Centre.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaban, S., Elsaeid, A.A., Ekram, B. et al. Curcumin loaded as prepared electrospun titania nanofibers for post breast cancer surgeries. J Sol-Gel Sci Technol 109, 346–361 (2024). https://doi.org/10.1007/s10971-023-06276-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06276-7