Abstract
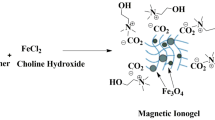
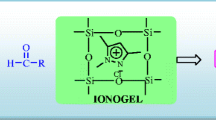
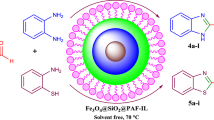
Despite the recent advancements in ionic liquids, incorporating these liquids into solid networks, particularly ionogels, to achieve new properties such as improved thermal and electrical conductivity and better mechanical and rheological attributes has been challenging. This study reports a novel magnetic ionogel using carbomer as a simple and cost-effective matrix. The catalytic activity of this new matrix was evaluated in a Knoevenagel reaction followed by a Michael addition, which resulted in synthesizing a diverse range of pyranopyrazole derivatives at room temperature in just 3–5 h. This approach was found to be tolerant of various functional groups and utilized low-cost reagents, leading to a robust synthesis and moderate to high yields ranging from 74 to 96%. The morphology of the magnetic ionogel was investigated through SEM and TGA spectroscopy. The catalyst was reused for up to five consecutive runs with excellent yield in a one-pot multicomponent reaction.
Graphical abstract

Highlights
-
Novel magnetic ionogel catalyst for one-pot synthesis of pyranopyrazole derivatives at ambient temperature.
-
Novel matrix with improved thermal, electrical conductivity and mechanical properties.
-
Tolerant of various functional groups, low-cost reagents, and moderate to high yields (74–96%).
-
Morphology investigated through SEM and TGA spectroscopy.
-
Catalyst reusable for up to five consecutive runs with excellent yield in one-pot multicomponent reaction.







Similar content being viewed by others
References
Gao F, Bai R, Ferlin F, Vaccaro L, Li M, Gu Y (2020) Replacement strategies for non-green dipolar aprotic solvents. Green Chem 22:6240–6257
Liu P, Hao JW, Mo LP, Zhang ZH (2015) Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv 5:48675–48704
Azizi N, Hasani M, Khajeh M, Edrisi M (2015) A straightforward and sustainable one-pot, four-component synthesis of rhodanine derivatives. Tetrahedron Lett 56:1189–1192
Suslick KS, Fang M, Hyeon T (1996) Sonochemical synthesis of iron colloids. J Am Chem Soc 118:11960–11961
Ma Z, Mohapatra J, Wei K, Liu JP, Sun S (2023) Magnetic Nanoparticles: Synthesis, Anisotropy, and Applications. Chem Rev 123:3904–3943
Martins PM, Lima AC, Ribeiro S, Lanceros-Mendez S, Martins P (2021) Magnetic Nanoparticles for Biomedical Applications: From the Soul of the Earth to the Deep History of Ourselves. ACS Appl Bio Mater 4:5839–5870
Gupta AK, Gupta M (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26:3995–402
Satoshi H, Hiroo H (2004) Discovery of a Magnetic Ionic Liquid [bmim] FeCl4. Chem Lett 33:1590–1592
Andrzejewska E, Marcinkowska A, Zgrzeba A (2017) Ionogels – materials containing immobilized ionic liquids. Polymer 62:344–352
Sutanto F, Shaabani S, Neochoritis CG, Zarganes-Tzitzikas T, Patil P, Ghonchepour E, Dömling A (2021) Multicomponent reaction-derived covalent inhibitor space. Sci Adv 7:abd9307
Mousavi H (2021) A comprehensive survey upon diverse and prolific applications of chitosan-based catalytic systems in one-pot multi-component synthesis of heterocyclic rings. Int J Biol Macromolecules 186:1003–1166
Mousavi H, Zeynizadeh B, Rimaz M (2023) Green and efficient one-pot three-component synthesis of novel drug-like furo[2,3-d]pyrimidines as potential active site inhibitors and putative allosteric hotspots modulators of both SARS-CoV-2 MPro and PLPro. Bioorg Chem 135:106390
Hasanpour Galehban M, Zeynizadeh B, Mousavi H (2022) NiII NPs entrapped within a matrix of L-glutamic acid cross-linked chitosan supported on magnetic carboxylic acid-functionalized multi-walled carbon nanotube: a new and efficient multi-task catalytic system for the green one-pot synthesis of diverse heterocyclic frameworks. RSC Adv 12:16454–16478
Hasanpour Galehban M, Zeynizadeh B, Mousavi H (2022) Diverse and efficient catalytic applications of new cockscomb flower-like Fe3O4@SiO2@KCC-1@MPTMS@CuII mesoporous nanocomposite in the environmentally benign reduction and reductive acetylation of nitroarenes and one-pot synthesis of some coumarin compounds. RSC Adv 12:11164–11189
Mousavi H (2022) A concise and focused overview upon arylglyoxal monohydrates-based one-pot multi-component synthesis of fascinating potentially biologically active pyridazines. J Mol Struct 1251:131742
Hasanpour Galehban M, Zeynizadeh B, Mousavi H (2023) Introducing Fe3O4@SiO2@KCC-1@MPTMS@CuII catalytic applications for the green one-pot syntheses of 2-aryl(or heteroaryl)-2,3-dihydroquinazolin-4(1H)-ones and 9-aryl-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-diones. J Mol Struct 1271:134017
Rimaz M, Mousavi H, Ozzar L, Khalili B (2019) Facile, capable, atom-economical one-pot multicomponent strategy for the direct regioselective synthesis of novel isoxazolo[5,4-d]pyrimidines. Res Chem Intermed 45:2673–2694
Dömling A (2006) Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem Rev 106:17–89
Sikandar S, Zahoor AF (2021) Synthesis of pyrano[2,3-c]pyrazoles: A review. J Heterocycl Chem 58:685–705
Myrboh B, Mecadon H, Rumum Rohman MD, Rajbangshi M, Kharkongor I, Laloo BM (2013) Synthetic Developments in Functionalized Pyrano[2,3-c]pyrazoles. A Review. Org Preparations Proced Int 4:253–303
Mamaghani M, Hossein Nia R (2021) A Review on the Recent Multicomponent Synthesis of Pyranopyrazoles. Polycycl Aromat Compd 41:223–291
Morgan LR, Jursic BS, Hooper CL, Neumann DM, Thangaraj K, Leblance B (2002) Anticancer activity for 4,4’-dihydroxybenzophenone-2,4-dinitrophenylhydrazone (A-007) analogues and their abilities to interact with lymphoendothelial cell surface markers. Bioorg Med Chem Lett 12:3407–11
Lian XZ, Huang Y, Li YQ, Zheng WJ (2008) A Green Synthesis of Tetrahydrobenzo [b] pyran Derivatives through Three-Component Condensation Using N-Methylimidazole as Organocatalyst. Monatshefte fur Chem 139:129–131
Kamble RD, Dawane BS, Yemul OS, Kale AB, Patil SD (2013) Bleaching earth clay (pH 12.5): a green catalyst for rapid synthesis of pyranopyrazole derivatives via a tandem three-component reaction. Res Chem Intermed 39:3859–3866
Devi I, Bhuyan PJ (2004) Sodium Bromide catalysed one-pot synthesis of tetrahydrobenzo [b] pyrans Via A three-component Cyclocondensation Under Microwave Irradiation and Solvent Free conditions. Tetrahedron Lett 45:8625–8627
Zheng J, Li Y (2011) Basic Ionic Liquid-Catalyzed One-Pot Synthesis of the Spiroacenaphthylene Derivatives in Water Medium. Mendeleev Commun 21:280–289
Khan AT, Lal M, Ali S, Khan MM (2011) One-pot three-component reaction for the synthesis of pyran annulated heterocyclic compounds using DMAP as a catalyst. Tetrahedron Lett 52:5327–5330
Shaterian HR, Kangani M (2014) Synthesis of 6-amino-4-aryl-3-methyl-1,4-dihydropyrano[2,3-c] pyrazole-5-carbonitriles by heterogeneous reusable catalysts. Res Chem Intermed 40:1997–2005
Kiyani H, Bamdad S (2018) Sodium ascorbate as an expedient catalyst for green synthesis of polysubstituted 5‑aminopyrazole‑4‑carbonitriles and 6‑amino‑1,4‑dihyd ropyrano[2,3‑c] pyrazole‑5‑carbonitriles. Res Chem Intermed 44:2761–2778
Khurana JM, Nand B, Saluja P (2010) DBU: a highly efficient catalyst for one-pot synthesis of substituted 3,4-dihydropyrano[3,2-c] chromenes, dihydropyrano[4,3-b] pyranes, 2-amino-4H-benzo[h]chromenes and 2-amino-4H benzo[g]chromenes in aqueous medium. Tetrahedron 66:5637–5641
Avudaiappan G, Unnimaya JT, Asha P, Unnikrishnan V, Sreekumar K (2011) Green synthesis of pyrazolopyranopyrimidinone and pyranopyrazole derivatives using porphyrin-initiated amine-functionalized PolyBCMO dendritic polymer as sonocatalyst. Tetrahedron Lett 51:5327–5332
Al-Matar HM, Khalil KD, Adam AY, Elnagdi MH (2010) Green One Pot Solvent-Free Synthesis of Pyrano[2,3-c] Pyrazoles and Pyrazolo[1,5-a] Pyrimidines. Molecules 15:6619–6629
Kondabanthini S, Katari NK, Srimannarayana M, Gundla R, Kapavarapu R, Pal M (2022) Wang resin catalyzed sonochemical synthesis of dihydropyrano[2,3-c]pyrazole derivatives and their interactions with SIRT1. J Mol Struct 1266:133527
Ghafuri H, Kazemnezhad Leili M, Esmaili Zand HR (2020) Copper-immobilized ionic liquid as an alternative to organic solvents in the one-pot synthesis of bioactive dihydropyrano[2,3-c]pyrazole derivatives. Appl Organomet Chem 34:E5757
Wu M, Feng Q, Wan D, Ma J (2013) CTACl AS catalyst for four-component, one-pot synthesis of pyranopyrazole derivatives in aqueous medium. Synth Commun 43:1721–1726
Paul S, Pradhan K, Ghosh SD, Das AR (2014) Uncapped SnO2 quantum dot catalyzed cascade assembling of four components: A rapid and green approach to the pyrano[2,3-c]pyrazole and spiro-2-oxindole derivatives. Tetrahedron 70:6088–6099
Dutta A, Rahman N, Kumar JE, Rabha J, Phukan T, Nongkhlaw R (2021) Catalyst-free UV365-assisted synthesis of pyran annulated heterocyclic scaffolds and evaluation of their antibacterial activities. Synth Commun 51:263–278
Thakur A, Tripathi M, Rajesh UC, Rawat DS (2013) Ethylenediammonium diformate (EDDF) in PEG600: An efficient ambiphilic novel catalytic system for the one-pot synthesis of 4H-pyrans via Knoevenagel condensation. RSC Adv 3:18142–18148
Yellapurkar I, Bhabal SR, Jangam K, Salve V, Patange S, More P (2021) Magnesium ferrichromate nanoparticles: an efficient and recyclable catalyst in the synthesis of pyrano[2,3-c]pyrazole derivatives. Res Chem Intermed 47:2669–2687
Nagaraju S, Paplal B, Sathish K, Giri S, Kashinath D (2017) Synthesis of functionalized chromene and spirochromenes using L-proline-melamine as highly efficient and recyclable homogeneous catalyst at room temperature. Tetrahedron Lett 58:4200–4204
Szczepańska K, Podlewska S, Dichiara M, Gentile D, Patamia V, Rosier N, Mönnich D, Ruiz Cantero MC, Karcz T, Łażewska D, Siwek A, Pockes S, Cobos EJ, Marrazzo A, Stark H, Rescifina A, Bojarski AJ, Amata E, Kieć-Kononowicz K (2022) Structural and Molecular Insight into Piperazine and Piperidine Derivatives as Histamine H3 and Sigma-1 Receptor Antagonists with Promising Antinociceptive Properties. ACS Chem Neurosci 13:1–15
Zhang RH, Guo HY, Deng H, Li J, Quan ZS (2021) Piperazine skeleton in the structural modification of natural products: a review. J Enzym Inhib Med Chem 36:1165–1197
Azizi N, Khajeh M, Alipour M (2014) Rapid and selective oxidation of alcohols in deep eutectic solvent. Ind Eng Chem Res 53:15561–15565
Azizi N, Saidi MR (2003) Lithium perchlorate diethyl ether solution: A highly efficient media for the abramov reaction. Phosphorus, Sulfur Silicon Relat Elem 78:1255–1259
Saidi MR, Azizi N (2003) Highly diastereoselective aminoalkylation of naphthols with chiral amines mediated by lithium perchlorate solution in diethyl ether. Tetrahedron Asymmetry 14:389–392
Azizi N, Shirdel F (2016) Task specific dicationic acidic ionic liquids catalyzed efficient and rapid synthesis of benzoxanthenones derivatives. J Mol Liq 222:783–787
Aamalzare M, Ahghari MR, Bayat M et al. (2021) Fe3O4@chitosan-tannic acid bionanocomposite as a novel nanocatalyst for the synthesis of pyranopyrazoles. Sci Rep. 11:20021
Beiranvand M, Habibi D (2021) Design, preparation and application of the semicarbazide-pyridoyl-sulfonic acid-based nanocatalyst for the synthesis of pyranopyrazoles. Sci Rep. 12:14347
Agrwal A, Pathak RK, Kasana V (2022) Molecular Docking and Antibacterial Studies of Pyranopyrazole Derivatives Synthesized Using [Pap-Glu@Chi] Biocatalyst Through a Greener Approach. Arab J Sci Eng 47:347–363
Dharmendra D, Chundawat P, Vyas Y, Chaubisa P, Kumawat M, Ameta C (2022) Eco-friendly design of TiO2 nanoparticles supported on Fe3O4 coated carbon-based biochar substrate for the synthesis of pyrano-[2, 3-c]-pyrazole derivatives. Sustain Chem Pharm 28:100732
Amiri-Zirtol L, Amrollahi MA (2022) Borax: An Environmentally Clean Catalyst for the Synthesize of Pyrano[2,3-c]Pyrazoles and Xanthene-1,8-Diones in H2O. Polycycl Aromat Compd 42:5696–5707
Ahmed MF, Mohammed AAE-N, Ahmed AE, Ali AS, Mohammad YA, Serag Eldin IE, Mohammed MA, Ahmed ME-A (2022) Synthesis of 1,4-dihydropyrano[2,3-c]pyrazole derivatives and exploring molecular and cytotoxic properties based on DFT and molecular docking studies. J Mol Struct 1249:131555
Kamanna K, Amaregouda Y (2022) Synthesis of bioactive scaffolds catalyzed by agro-waste-based solvent medium. Phys Sci Rev https://doi.org/10.1515/psr-2021-0097 (2022).
Patil UP, Patil RC, Patil SS (2019) An Eco-friendly Catalytic System for One-pot Multicomponent Synthesis of Diverse and Densely Functionalized Pyranopyrazole and Benzochromene Derivatives. J Heterocycl Chem 56:1898–1913
Parmar MP, Vala RM, Patel HM (2023) Importance of Hybrid Catalysts toward the Synthesis of 5 H-Pyrano[2,3-d]pyrimidine-2-ones/2,4-diones (Thiones). ACS Omega 8:1759–1816. https://doi.org/10.1021/acsomega.2c05349
Mokariya JA, Rajani DP, Patel MP (2022) Anomaly of Pyrano[2,3‐c]pyrazole Synthesis towards Pyrazolyl‐aryl‐methyl‐malononitrile Derivatives and Their Antimicrobial Activity. Chem Select 7:e202201341
Theresa LV, Pradeep SD, Sebastian D, Sreekumar K (2021) Sustainable multicomponent one pot synthesis of pyranopyrazole derivatives in the presence of Lactic acid: Urea: NH4Cl. Current Research in Green and Sustainable. Chemistry 4:100194
Mishra M, Jomon KJ, Sriram Krishnan VR, Nizam A (2020) [18-C-6H3O+]: an in-situ generated macrocyclic complex and an efficient, novel catalyst for synthesis of pyrano[2,3-c]pyrazole derivatives. Sci Rep 10:14342
Chowhan B, Kour J, Gupta M, Paul S (2021) Green Synthesis of Bis(pyrazol‐5‐ole) and Pyrazolopyranopyrimidine Derivatives through Mechanochemistry Using Chitosan as a Biodegradable Catalyst. Chem Select 6:7922–7930
Acknowledgements
The authors acknowledge support from the Chemistry and Chemical Engineering Research Center of Iran and the Iran National Science Foundation (INSF), under project No.4013509.
Author contributions
SS carried out data collection and analysis. NA wrote the first draft of the manuscript, ZM supervised, and HS reviewed and edited the final draft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shojaee, S., Azizi, N., Mirjafary, Z. et al. Fabrication of novel magnetic ionogel catalyst for one-pot synthesis of pyranopyrazole derivatives at ambient temperature. J Sol-Gel Sci Technol (2023). https://doi.org/10.1007/s10971-023-06214-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10971-023-06214-7