Abstract
As a result of the global demand for sustainable products, a suitable alternative to the resorcinol-formaldehyde aerogels, which are frequently used as precursors for carbon aerogels, is searched for. In this study, the replacement of petroleum-derived formaldehyde with a natural, biobased crosslinker, namely 5-(hydroxymethyl)furfural (5-HMF) is shown, and the synthesis of renewable, monolithic tannin aerogels is demonstrated. Compared to well-known tannin-formaldehyde aerogels, this green alternative shows lower reactivity of the crosslinker associated with lower gelation times as well as lower specific surface areas at the organic stage. Nonetheless, the morphologies and synthesis-structure relationships follow similar trends for both tannin-based aerogels, e.g., the pore size is influenced by the initial pH in the same manner. The turnover to carbon aerogels by a carbothermal treatment results in enhanced high-specific surface areas of the tannin-5-HMF-based carbon aerogels, which are similar and even slightly outperform those obtained from tannin-formaldehyde aerogels. This suggests that they are a convenient alternative for carbon aerogel applications.
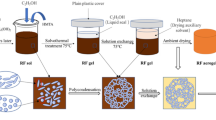
Graphical Abstract

Circular scheme of the formaldehyde-free, synthetic pathway to organic and related carbon aerogels based on renewable sources: mimosa tannin and 5-(hydroxymethyl)furfural.
Highlights
-
Renewable tannin-5-(hydroxymethyl)furfural (TH) aerogels are synthesized via sol–gel processing.
-
The replacement of formaldehyde by biomass-derived 5-HMF is demonstrated.
-
TH aerogels are found to be a convenient, green alternative to tannin-formaldehyde (TF) aerogels as carbon precursor material with similar/even slightly outperforming properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Aerogels are three-dimensional, nanoporous materials that feature exceptional properties, such as low-density and high-specific surface area [1]. Particularly, organic polymeric aerogels, with resorcinol-formaldehyde (RF) being the most predominate representative, are of great interest not only due to their typical aerogel properties, but also due to the ease of conversion to the corresponding carbon counterparts. This enables them to be used for a wide range of applications, such as absorbents or porous electrodes [2]. Furthermore, applications such as energy storage devices (e.g., supercapacitors [2]), hydrogen storage [3, 4], carbon dioxide capture [5] as well as water desalination [6] are presently of extraordinary interest. The high reactivity and simple chemistry of RF, in combination with the option to tailor the final morphology of the final gel by simply changing the synthesis pH value, precursor, or temperature, make them attractive for the preparation of carbon aerogels [7]. However, severe drawbacks of using RF include high costs and the dependency on petroleum-based and harmful precursors [2, 8]. Targeting the sustainability and cost aspects of commercial materials, polyphenolic tannins were found to present a suitable eco-friendly, low-cost carbon source [9]. As a promising alternative to RF aerogels, the synthesis of tannin-formaldehyde (TF) gels has seen significant interest as a precursor in the production of carbon aerogels [2, 10,11,12]. However, in the majority of studies regarding tannin-based aerogels, toxic, carcinogenic formaldehyde as a crosslinker is applied. By employing an eco-friendly product policy, the replacement of formaldehyde with other less toxic and harmful reagents is demanded. Most research dedicated to this issue involves glutaraldehyde, glyoxal and hexamine, which however feature a much weaker crosslinking efficiency than formaldehyde, thus strongly affecting the formation of a stable gel [13]. The biomass-derived 5-(hydroxymethyl)furfural (5-HMF) [14,15,16] has been examined for the preparation of tannin wood adhesives due to the two reactive sites of 5-HMF. More precisely, this furan derivate not only possesses the reactive aldehyde group but rather also features a reactive hydroxymethyl group, which is located on the furan ring [17].
In this study, the usage of biomass-derived, non-toxic 5-HMF as a crosslinker for mimosa tannin-based aerogels is explored. Featuring solely sustainable precursors in a sol–gel-based approach, organic aerogels are prepared for the first time and the effects of initial pH and amount of solvent (dilution) on their physical properties are evaluated. In addition, the transition to carbon aerogels by simple thermal treatment in inert atmosphere is shown. The focus of the study is a direct comparison of these two tannin-based gel carbon precursor systems to assess the suitability of replacing formaldehyde with the biomass-derived, non-toxic 5-HMF.
2 Materials and methods
2.1 Chemicals
Technical grade acetone (96%), technical grade methanol (>98.5%), and sodium hydroxide pellets were provided by VWR. Hydrochloric acid (37%) and formaldehyde (37% in water, stabilized with 10% methanol) were supplied by Merck. 5-HMF (≥95%) was acquired from AVA Biochem (Switzerland). As the source of condensed flavonoid tannin, a commercially available mimosa extract (Weibull AQ) from the company Tanac (Brazil) was used.
2.2 Synthesis of tannin-formaldehyde and tannin-5-(hydroxymethyl)furfural aerogels
The synthesis of TF organic wet gels was implemented in accordance with Szczurek et al. [2]. TF aerogels with a theoretical density of 0.1 g cm−3 (determined as ρtheo = m(tannin)/VSol) were prepared by mixing 1.25 g mimosa tannin with 9.75 g of a 20% aqueous methanol solution. Then, 2.5 g (30.8 mmol) of formaldehyde (37%) was added to the solution. The pH value of the solution was adjusted to 3, 5, 7 and 9 by either using hydrochloric acid (1 M) or sodium hydroxide (0.1 M). The sol was stirred for 10 min and subsequently poured in cylindrical glass vessels and placed in an oven at 80 °C to promote gelation. After 7 days of aging, the gels were removed from the glass vials and washed in acetone (24 h cycle, five times, 50 ml). Afterwards, the wet gels were dried using supercritical extraction with CO2 (60 °C, 110 bar) [18]. In addition, to the above-named formulation, TF aerogels with a theoretical density of 0.05 g cm−3 have been prepared using 0.625 g tannin, 1.25 g (15.4 mmol) formaldehyde, and 10.85 g 20% aqueous methanol solution.
Tannin-5-HMF (TH) aerogels were synthesized accordingly to the TF aerogels, whilst solely replacing formaldehyde with 5-HMF. The amount of substance was kept equal. Thus, for the preparation of TH aerogels with a theoretical density of 0.1 g cm−3, 1.25 g tannin, 3.88 g (30.8 mmol) 5-HMF, and 9.75 g 20% aqueous methanol solution were used. The amounts used for the preparation of TH aerogels with a theoretical density of 0.05 g cm−3 account 0.625 g tannin, 1.94 g (15.4 mmol) 5-HMF and 10.85 g 20% aqueous methanol solution.
The obtained organic TF and TH gels were carbonized in a tube furnace (800 °C, heating rate 60 °C h−1, dwell time 2 h, 75 NL h−1 argon flow).
2.3 Characterization
The bulk density ρb (g cm−3) was determined in duplicate by weighing a cylindrical sample of known dimensions and dividing it by its geometrical volume.
The diametric shrinkages of the gels after supercritical drying were recorded in duplicates by measuring the samples’ diameter after supercritical drying and comparing it with the inner diameter of the PP containers. The diametric shrinkage of the carbonized gels was determined by comparing their diameter with that recorded for the aerogels after supercritical drying.
The skeletal density ρs (g cm−3) was obtained by analyzing small pieces of the monoliths using helium pycnometry, which was performed on a ULTRAPYC 1200 e automatic density analyzer (Quantachrome instrument). Determination of the bulk and skeletal density allows the calculation of the overall porosity Φ of the sample according to the following formula:
Nitrogen physisorption isotherms were recorded on a Sy-Lab Micromeritics ASAP 2420 surface area and porosity analyzer at −196 °C and in a relative pressure range p/p0 from 10−7 to 1. Prior to analysis, the samples were degassed under vacuum (24 h, 80 °C). The specific surface area SBET of the (carbon) tannin-based aerogels was determined using the Brunauer, Emmett, and Teller method [19]. Furthermore, the pore size distribution for the nitrogen isotherm’s desorption branch has been analyzed by using the Barrett, Joyner and Halenda method [20].
The morphology of the tannin-based samples was analyzed by scanning electron microscopy (SEM), recorded with a Zeiss Ultra Plus instrument, using an in-lens secondary electron detector as well as a varying acceleration voltage (5–10 kV). The samples were prepared on a carbon tape and the organic tannin samples were sputtered with gold (90 s, 40 mA).
The carbonized tannin-based aerogels were analyzed using Raman spectroscopy. The Raman spectra were collected on a Thermo Scientific DXR2 Raman microscope, which was equipped with a confocal microscope BX41 (Olympus Corp.) and a ×10 objective, delivering a laser spot diameter of ~2 µm, and a 50 µm pinhole-like entrance slit to the spectrometer. Furthermore, the Raman spectra were acquired with a 532 nm laser excitation wavelength and a laser power of 4 mW in the range of 500–3500 cm−1.
3 Results and discussion
3.1 Synthesis of organic TF and TH aerogels
TF aerogels are now well-discussed in the literature [2, 11], valorizing tannin as a suitable, sustainable, abundant and non-toxic carbon source for the partial replacement of classical raw-oil-based RF aerogels. However, according to more severe regulations concerning the use and emission of toxic formaldehyde, non-toxic bio-sourced natural alternatives for formaldehyde have also to be considered [17]. In order to evaluate the potential of 5-HMF as a green crosslinker alternative to formaldehyde for the generation of tannin-based (carbon) aerogels, a series of tannin-5-HMF (TH) as well as TF aerogels with constant tannin/crosslinker molar ratio and theoretical densities of 0.05 and 0.1 g cm−3 were prepared. Additionally, the influence of various catalyst concentrations (pH values of 3–9) was investigated to study synthesis-morphology relationships.
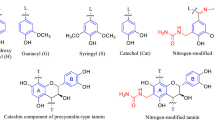
The proposed polycondensation reaction of mimosa tannin with either formaldehyde or 5-HMF is demonstrated in Fig. 1. Generally, the reaction of condensed tannins with aldehydes mainly takes place at the tannins’ A-rings C6 or C8 sites [21,22,23]. When reacting tannin with formaldehyde, methylene bridges or methylene-ether bridges form a crosslinked network with potential release of excess formaldehyde [24]. In order to avoid the use of formaldehyde and its release during the polymerization reaction, the usage of a biobased (aldehyde) crosslinker, which leads to no or only low-level formaldehyde release during the synthesis, is preferred. However, alternative aldehydes have the disadvantage of a lower reactivity compared to formaldehyde [17]. In contrast, 5-HMF has two functional groups, which can react in the polycondensation reaction with condensed tannins, namely the furanic aldehyde group and the furanic hydroxymethyl group. In a neutral or alkaline environment, mainly the aldehyde group reacts yielding a three-dimensional TH network structure as shown in Fig. 1. In contrast, the hydroxymethyl group is expected to be highly reactive under very acidic or alkaline conditions or at elevated temperatures [17].
In order to determine the reactivity of the utilized crosslinker toward tannin, the gelation time was monitored (Fig. 2). Gelation of TF aerogels took place within several hours, whereas TH aerogels gelled within several days. Thus, in general, tannins show a distinctly greater reactivity toward formaldehyde compared to 5-HMF. This can be attributed to larger diffusion constraints and stoichiometric hindrance of the 5-HMF molecule, containing six carbon atoms compared to one carbon atom of formaldehyde [22]. Nevertheless, the reactivity in the gelation process can deliberately be tailored either by dilution (theoretical density) or by pH adjustment (catalyst concentration). First, at higher monomer concentrations (larger theoretical density) shorter gelation times are obtained due to a higher amount of condensable species in a given volume. Furthermore, the gel time is slowest at tannins’ natural pH value (~4.3) and the condensation velocity can be increased by a catalyzed environment (either acidic or basic), resulting in distinctly faster gelation times [11]. More precisely, TF aerogels showed the shortest gelation time (0.9 h) under acid-catalyzed conditions (pH 3). Besides, base-catalyzed conditions (pH 7 and 9) also show decreased gel times, whereas higher pH values (pH 9) result in even lower gel times. Nevertheless, in general an acidic-catalyzed medium triggers faster gelation compared to an alkaline-catalyzed medium. Likewise, TH aerogels gel faster in a catalyzed environment. However, stronger alkaline conditions (pH 9) yield the shortest gel times with 2 d (theoretical density of 0.1 g cm−3) compared to weaker base-catalyzed conditions (pH 7) or acidic conditions (pH 3). It has to be noted that at a theoretical density of 0.05 g cm−3 and a pH value of 3 no gelation occurred. Due to the longer gelation times for TH aerogels, it is assumed that mainly the aldehyde group participates in the polycondensation reaction, being in good agreement with its expected lower reactivity compared to formaldehyde. However, presumably, the hydroxymethyl group also reacts at the alkaline pH value (pH 9) due to the distinctly shorter gelation time observed under these conditions. Moreover, this pH-dependent gelation behavior is also observed for RF gels, as the maximum gelation time is recorded at a pH of roughly 4 and the use of an acid or base catalyst enhances the gelation rate [25].
3.2 Structural characterization of organic TF and TH aerogels
TF and TH aerogels do not only differ in their gelation times but also in their visual appearance, as illustrated in Figs. 3 and 4, respectively. The aerogels differ distinctly in color, depending on the theoretical density and pH value. In particular, an increasing theoretical density (higher tannin concentration) results in an increased color intensity of the gels, accounting for smaller particle sizes. Similarly, higher pH values also contribute toward smaller particle sizes [11]. Additionally, the difference in the color of the monoliths might be provoked by oxidation reactions of the free hydroxyl groups of the three-dimensional tannin network, which thus might result in an increase in the absorption range of the molecule in the visible spectrum. More precisely, gels, prepared at a higher pH, feature a network with more free hydroxyl groups and hence a wider wavelength range is absorbed yielding a darker brownish color. In contrast, an acidic pH value yields a light brownish gel. Furthermore, the presence of quinones, produced by oxidation of phenolic compounds, within the network structure is suggested in the literature [11]. It is known, that the color of quinones changes from red, brown to almost black, when changing the pH from acidic to alkaline [11]. Both aerogel systems (TF and TH) thereby follow similar trends. However, the TH aerogels overall feature darker colors compared to the TF aerogels, suggesting differences in the network structure and/or particle sizes.
The presumed differences in particle size distributions, which affect the light transmission through the gel, are further investigated by SEM of both the TF and TH aerogels with a theoretical density of 0.1 g cm−3 (Fig. 5). In general, the networks’ particle size is decreasing with an increasing pH value, resulting in the darker color of the monoliths synthesized at a higher pH value. In accordance with the same pH trend, a distinct loss of macropores is observed in the network for both aerogel series. Moreover, TH aerogels feature visually slightly smaller particle sizes compared to TF aerogels, again explaining the color differences.
The aerogels, prepared with a theoretical density of 0.05 g cm−3, show a similar effect, where an increasing pH value yields decreasing particle sizes (Supporting Information, Fig. S1). Similarly, the particle size of RF gels is also controlled by the catalyst concentration (pH value), as a high alkaline catalyst concentration provokes the formation of small network clusters during the gelation process [25].
Apart from changes in the appearance and morphology of the TF and TH aerogels, the diametric shrinkage, bulk density, and porosities are influenced by the initial pH value and the applied precursor dilution (Table 1). For both the TF and TH aerogels, the diametric shrinkage increases with an increasing pH value. Furthermore, a lower theoretical density (0.05 g cm−3) results in higher shrinkage at equal pH values, implying lower network stability of the gels prepared at high dilutions and hence leading to some collapse during the drying process [11]. TF aerogels show lower shrinkage values, ranging from 21.7–30.7% at the pH values of 3, 5, and 7, for a theoretical density of ρtheo 0.1 g cm−3 compared to the TH aerogels with shrinkage values of 24.0–37.2%. In contrast to that, TH aerogels feature slightly lower shrinkage values at higher dilutions with 31.2–47.8% (pH 5–9) compared to TF aerogels with 33.6–57.5% (pH 5–9). In general, the shrinkage values between the two systems vary slightly while following the same trends. Likewise, for wet RF gels it was found that gels with smaller particles, hence prepared at a higher pH, have higher shrinkage values after supercritical drying, due to incomplete water removal during the solvent exchange leading to a higher residual surface tension. However, on the contrary to the TH and TF gels, higher shrinkage values are observed for lower dilutions (higher theoretical density) [25].
Similar behavior is determined for the bulk density, which directly correlates with the diametric shrinkages. In particular, the higher shrinkage of the aerogels yields a higher bulk density. Hence, the bulk densities of the TF aerogels with theoretical densities of 0.05 g cm−3 and 0.1 g cm−3 account for 0.09–0.62 and 0.17–0.57 g cm−3, respectively. It has to be noted that the bulk density of the TF aerogel, prepared at a higher theoretical density and a pH value of 9, is even lower than the one prepared at a lower theoretical density due to the decrease of diametric shrinkage at higher theoretical densities. Likewise, the bulk densities of the TH aerogels with theoretical densities of 0.05 g cm−3 and 0.1 g cm−3 were 0.20–0.45 and 0.26–0.60 g cm−3. Moreover, the porosity of the aerogels is increasing with decreasing shrinkages, corresponding to a decreasing pH value. Overall, the porosity of the TF and TH aerogels solely vary slightly with porosities of 94.2–62.1% and 88.9–62.3%, respectively.
The specific surface areas (SBET) and pore size distributions (according to BJH) were determined from nitrogen adsorption measurements (Table 1). The obtained isotherms of the TF and TH aerogels with theoretical densities of 0.1 g cm−3 are depicted in Fig. 6. According to the IUPAC classification [26], the isotherms of the aerogels are of Type IV, suggesting the presence of mainly mesopores. This physisorption isotherm type features a characteristic hysteresis loop, which indicates the capillary condensation effect within the mesopores (above p/p0 of 0.5) [27]. The type of hysteresis loop, depending on the adsorption mechanism correlates with the pore structure. For both, TF and TH aerogels, prepared at a pH value of 3–7, the hysteresis loop is of type H3, which is characteristic of a very wide pore distribution, which can be seen in the Supporting Information in Fig. S2. In contrast, aerogels prepared in very alkaline medium (pH 9) feature H2 type hysteresis loops, corresponding to ink-bottle shape pores with a distinct pore size [11]. Furthermore, the isotherms of the aerogels, prepared at a pH value of 3–7 show no saturation of N2 uptake at relative pressure close to unity, indicating the presence of macropores (>50 nm), which is in good agreement with the SEM images (Fig. 5) [26]. On the contrary, the aerogels, prepared at a pH of 9, show a leveling at high relative pressures, indicating a purely mesoporous system. This behavior is also overserved for RF gels, as the formation of small particles at higher alkaline catalyst concentrations yields a network with smaller pores [25].
According to BJH analysis of the desorption branch, the TF and TH aerogels feature the smallest pore sizes below 10 nm, when working in an alkaline environment (pH 9), which is in accordance with literature values for phenolic aerogels [11]. In general, TF aerogels, prepared at a theoretical density of 0.1 g cm−3 show slightly lower pore sizes, ranging from 7.5–11.9 nm compared to TH aerogels (9.7–16.0 nm), suggesting slight structural differences between these two systems, as already presumed from the SEM images. Apart from differences in the actual pore sizes, deviations in the specific surface areas are observed, whereas TF aerogels feature distinctly higher SBET values varying from 626–917 m2 g−1 compared to TH aerogels with surface areas of 364–589 m2 g−1. These differences in specific surface areas arise due to the significantly larger micropore volume of TF aerogels compared to TH aerogels.
Similar trends are observed for the sorption isotherms of the TF and TH aerogels, prepared at a theoretical density of 0.05 g cm−3 (Supporting Information, Fig. S3). However, the TH aerogel, prepared at pH 9 show a type H3 hysteresis loop in this case, suggesting a wide pore size distribution. The values for the specific surface area remain similar, but the pore sizes generally shift to slightly larger pore sizes.
3.3 Analysis of TF- and TH-based carbon aerogels
Organic aerogels very often serve as precursors for the analogous carbon replica, generated by carbonization in inert atmosphere. The carbothermal treatment of TF and TH aerogels and their resulting properties were investigated and all aerogels were successfully carbonized (Fig. 7).
The TH aerogels feature 23.1–28.0% diametric shrinkages after the carbonization process, which are lower than the corresponding values for the TF aerogels, which show 26.4–32.7% shrinkage (Table 2). However, since the TH organic aerogels had higher bulk densities, they still feature, even after the carbonization process with lower shrinkages, higher bulk densities for the carbon aerogel, initially prepared at a pH value of 3–7. More precisely, TH carbon aerogels have resulting bulk densities in the range of 0.28–0.82 g cm−3 while TF carbon aerogels feature values ranging from 0.21 to 0.96 g cm−3 (pH 3–9).
SEM images of the carbonized aerogels were recorded and are illustrated in Fig. 8. The trend of decreasing particle size with increasing pH value for both TF and TH carbon aerogels, is similar to the organic tannin-based aerogels (Fig. 5). Thus, no change in microstructure is observed after the carbonization process. To further investigate the pore structure of the carbon aerogels, nitrogen sorption experiments were performed.
The nitrogen physisorption isotherms of the carbonized TF and TH aerogels (ρtheo 0.1 g cm−3) are illustrated in Fig. 9 and their pore size distributions are shown in the Supporting Information in Fig. S4. The carbonized TF and TH aerogels show similar behavior regarding the adsorption of nitrogen, exhibiting a type IV isotherm with a characteristic hysteresis loop of type 3 (pH 3–7) and type 2 (pH 9) indicating the presence of pores in the mesopore range. As expected, the pore size distribution remains similar with the smallest pore sizes of 7.0 nm and 9.4 nm for the TF and TH aerogels, respectively, prepared at pH 9. Similar to the organic aerogels, the carbon aerogels, prepared at an initial pH value of 9 show a saturation of N2 uptake at a relative pressure close to unity, indicating the absence of macropores. Furthermore, a relevant nitrogen uptake at very low relative pressure for both carbon aerogels (TF as well as TH) is observed, and hence the existence of micropores can be assumed. However, the increase of nitrogen uptake at low relative pressures is distinctly larger for the TH aerogels after carbonization (average uptake in micropore volume of 0.14 cm3 g−1), compared to the TF aerogels (average uptake in micropore volume of 0.05 cm3 g−1), which shows nearly no additional nitrogen uptake at low relative pressures after carbonization. In particular, a greater amount of micropores is generated in the TH network compared to the TF network during the carbonization process. Hence, the carbonization process is predicted to influence the specific surface area of the resulting material. For the TF aerogels, the surface area remains constant or even decreases slightly after the carbonization process to 587–712 m2 g−1, whereas a lower pH value yields higher surface areas. In contrast, SBET of the TH aerogels increases considerably to 694–825 m2 g−1, which presumably accounts for the increase in present micropores. Hence, after carbothermal treatment, TH aerogels are highly promising candidates as biopolymeric carbon aerogel precursor material with similar and even slightly superior properties compared to the commercially applied environmentally-harmful TF carbon precursors.
Moreover, the carbon structure of the TH and TF-based carbon aerogels has been analyzed using Raman spectroscopy. The resulting Raman spectra are shown in Fig. 10. All Raman spectra display the characteristic carbon D-band (roughly 1343 cm−1) and G-band (roughly 1595 cm−1). The D-band corresponds to the sp2-hybridized disordered carbon (A1g in-plane breathing vibration mode) and the G-band corresponds to the ordered graphite (E2g in-plane vibration mode) [28, 29]. Overall, in a pure graphitic structure, only the G-band is present, whereas the D-band becomes visible with an increase in disordered carbon structure. Thus, the material’s relative graphitization degree is given as the ratio of the D- and G-band areas (AD/AG ratio), where a higher value indicates a more disordered structure [28, 30]. All carbonized tannin-based aerogels show AD/AG peak area ratios between 2.8 and 3.3, implying a similar incomplete crystalline character with a large contribution of amorphous carbon (Table S1) [29]. Moreover, additionally to the AD/AG ratio, also the broadening of the D- and G-bands suggests a disordered carbon structure. Due to this band broadening the Raman spectra of the TF and TH carbon aerogels were fitted by deconvolution into five bands, namely D*, D, D**, G, and D’ (Supporting Information, Figs. S5 and S6, respectively) [31]. The D* (roughly 1192 cm−1) and D** bands (roughly 1531 cm−1) are observed with a significant contribution (typical signature of existing edge effects by oxygen, in plane defects or tetrahedral carbon atoms) in both the carbonized TF and TH aerogel samples in addition to the conventional D- and G-bands, and hence imply a disordered carbon structure [32].
4 Conclusion
A promising, green-chemistry-based route to monolithic tannin-5-HMF (TH) aerogels and related carbon aerogels is presented and is compared in a synthesis-structure relationship to TF aerogels. In terms of reaction kinetics, TF aerogels are superior due to the higher reactivity of formaldehyde in the condensation of tannins. More precisely, TF aerogels gel within hours, whereas TH aerogels need several days for gelation to occur. Thus, according to the different polycondensation reaction rates, different network structures are obtained with slightly smaller particle sizes for the TH aerogels compared to the TF aerogels. Furthermore, the particle sizes follow similar trends by adjusting the initial pH value, whereas a higher pH value yields smaller particle sizes. Additionally, the pH value also influences the pore size distribution. Samples prepared at pH values of 3, 5, or 7 show a meso- macroporous network with a broad pore size distribution, while samples prepared at pH 9 yield a narrowly distributed mesopore size. TF aerogels generally show slightly smaller pore size distributions compared to TH aerogels. Moreover, also significant differences in the specific surface area are observed with TF aerogels having distinct higher surface areas of roughly 626–917 m2 g−1 compared to TH aerogels with 364–589 m2 g−1. After carbonization, however, the TH aerogels feature similar or even higher specific surface areas compared to TF aerogels, making them hence highly suitable as a green precursor material for highly porous carbons.
Overall, tannin-5-HMF aerogels emerge as a promising biopolymer alternative to commercially available TF aerogels, particularly regarding to their conversion to carbon aerogels. Further possible tuning of the pore structure, e.g., by carbon dioxide activation will be investigated with the intention that these bio-based carbon aerogels become promising candidates for various carbon aerogel applications in the future.
References
Hüsing N, Schubert U (2000) Aerogels. In: Elvers B (ed.), Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH Verlag GmbH & Co. KGaA (Weinheim/Germany), p 631–640
Szczurek A, Amaral-Labat G, Fierro V et al. (2011) The use of tannin to prepare carbon gels. Part I: carbon aerogels. Carbon N Y 49:2785–2794. https://doi.org/10.1016/j.carbon.2011.03.005
Yu S, Song S, Li R, Fang B (2020) The lightest solid meets the lightest gas: an overview of carbon aerogels and their composites for hydrogen related applications. Nanoscale 12:19536–19556. https://doi.org/10.1039/d0nr05050d
Kabbour H, Baumann TF, Satcher JH et al. (2006) Toward new candidates for hydrogen storage: high-surface-area carbon aerogels. Chem Mater 18:6085–6087. https://doi.org/10.1021/cm062329a
Gan G, Li X, Fan S et al (2019) Carbon aerogels for environmental clean-up. Eur J Inorg Chem 2019:3126–3141. https://doi.org/10.1002/ejic.201801512
Kumar R, Sen Gupta S, Katiyar S et al. (2016) Carbon aerogels through organo-inorganic co-assembly and their application in water desalination by capacitive deionization. Carbon N Y 99:375–383. https://doi.org/10.1016/j.carbon.2015.12.004
Tamon H, Ishizaka H, Araki T, Okazaki M (1998) Control of mesoporous structure of organic and carbon aerogels. Carbon N Y 36:1257–1262. https://doi.org/10.1016/S0008-6223(97)00202-9
Rey-Raap N, Szczurek A, Fierro V et al. (2016) Advances in tailoring the porosity of tannin-based carbon xerogels. Ind Crops Prod 82:100–106. https://doi.org/10.1016/j.indcrop.2015.12.001
Arenillas A, Menéndez JA, Reichenauer G et al (2019) Organic and carbon gels. Springer Nature, Switzerland AG, Cham
Amaral-Labat G, Grishechko LI, Fierro V et al. (2013) Tannin-based xerogels with distinctive porous structures. Biomass Bioenergy 56:437–445. https://doi.org/10.1016/j.biombioe.2013.06.001
Amaral-Labat G, Szczurek A, Fierro V et al. (2013) Systematic studies of tannin-formaldehyde aerogels: preparation and properties. Sci Technol Adv Mater 14:015001. https://doi.org/10.1088/1468-6996/14/1/015001
Rey-Raap N, Arenillas A, Menéndez JA (2015) Formaldehyde in the synthesis of resorcinol-formaldehyde carbon gels. In: Patton A (ed.), Formaldehyde: synthesis, applications & potential health effects. Nova Science Publisher (NewYork, NY, USA), p 30–60
Szczurek A, Fierro V, Medjahdi G, Celzard A (2019) Carbon aerogels prepared by autocondensation of flavonoid tannin. Carbon Resour Convers 2:72–84. https://doi.org/10.1016/j.crcon.2019.02.001
Menegazzo F, Ghedini E, Signoretto M (2018) 5-Hydroxymethylfurfural (HMF) production from real biomasses. Molecules 23:1–18. https://doi.org/10.3390/molecules23092201
Snyder FH (1958) Preparation of hydroxymethylfurfural from cellulosic materials
Rapp KM (1988) Process for preparing pure 5-hydroxymethylfurfuraldehyde
Santiago-Medina F-J, Pizzi A, Abdalla S (2017) Hydroxymethylfurfural hardening of pine tannin wood adhesives. J Renew Mater 5:435–447. https://doi.org/10.7569/JRM.2017.634166
Selmer I, Behnecke AS, Quiño J et al. (2018) Model development for sc-drying kinetics of aerogels: Part 1. Monoliths and single particles. J Supercrit Fluids 140:415–430. https://doi.org/10.1016/j.supflu.2018.07.002
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319. https://doi.org/10.1021/ja01269a023
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73:373–380. https://doi.org/10.1021/ja01145a126
Arbenz A, Avérous L (2015) Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem 17:2626–2646. https://doi.org/10.1039/c5gc00282f
García DE, Glasser WG, Pizzi A et al. (2014) Polyphenolic resins prepared with maritime pine bark tannin and bulky-aldehydes. Ind Crops Prod 62:84–93. https://doi.org/10.1016/j.indcrop.2014.08.010
Shirmohammadli Y, Efhamisisi D, Pizzi A (2018) Tannins as a sustainable raw material for green chemistry: a review. Ind Crops Prod 126:316–332. https://doi.org/10.1016/j.indcrop.2018.10.034
Braghiroli FL, Amaral-Labat G, Boss AFN et al (2019) Tannin gels and their carbon derivatives: a review. Biomolecules 9. https://doi.org/10.3390/biom9100587
Elkhatat AM, Al-Muhtaseb SA (2011) Advances in tailoring resorcinol-formaldehyde organic and carbon gels. Adv Mater 23:2887–2903. https://doi.org/10.1002/adma.201100283
Sing KSW, Everet DH, Haul RAW (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 57:603–619
Thommes M, Kaneko K, Neimark AV et al (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069. https://doi.org/10.1515/pac-2014-1117
Wassner M, Eckardt M, Reyer A et al. (2020) Synthesis of amorphous and graphitized porous nitrogen-doped carbon spheres as oxygen reduction reaction catalysts. Beilstein J Nanotechnol 11:1–15. https://doi.org/10.3762/bjnano.11.1
Koopmann A-K, Torres-Rodríguez J, Salihovic M et al. (2021) Tannin-based nanoscale carbon spherogels as electrodes for electrochemical applications. ACS Appl Nano Mater 4:14115–14125. https://doi.org/10.1021/acsanm.1c03431
Barbera K, Frusteri L, Italiano G et al. (2014) Low-temperature graphitization of amorphous carbon nanospheres. Chin J Catal 35:869–876. https://doi.org/10.1016/s1872-2067(14)60098-x
Kaniyoor A, Ramaprabhu S (2012) A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv 2:032183. https://doi.org/10.1063/1.4756995
Coccato A, Jehlicka J, Moens L, Vandenabeele P (2015) Raman spectroscopy for the investigation of carbon-based black pigments. J Raman Spectrosc 46:1003–1015. https://doi.org/10.1002/jrs.4715
Acknowledgements
The authors acknowledge Clarisse Caliman (University of Salzburg) for performing preliminary experiments.
Funding
The research was financially supported by the Salzburg Center for Smart Materials (P1727558-IWB01), which is funded by the European Funds for Regional Development (EFRE) and the Austrian Wirtschaftsservice (AWS). Furthermore, TB gratefully acknowledges the financial support from the Austrian Science Fund (FWF, project no. P-33159). The authors acknowledge the financial support for the DXR2 Raman microscope provided by the European Regional Development Fund and Interreg V-A Italy-Austria 2014–2020 through the Interreg Italy-Austria project ITAT 1023 InCIMa “Smart Characterization of Intelligent Materials” and the Interreg Italy-Austria project ITAT1059 InCIMa4 for Science and SMEs project. Open access funding provided by Paris Lodron University of Salzburg.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by A-KK and TB. The first draft of the manuscript was written by A-KK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koopmann, AK., Bartschmid, T., Hüsing, N. et al. Renewable, Organic and Related Carbon Aerogel Monoliths from the Polycondensation of Tannin with 5-(Hydroxymethyl)furfural. J Sol-Gel Sci Technol (2023). https://doi.org/10.1007/s10971-022-06015-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10971-022-06015-4