Abstract
Water electrolysis can be used to generate hydrogen, petrochemical fuel with high efficiency for use in power generation and a high gravimetric energy density that can be utilized to battle the exhaustion and pollution produced by current fossil fuels. The use of transition metal chalcogenides (TMC) as a potential alternative to precious metals in the water splitting process has recently sparked much attention. Hence, developing the future of the hydrogen economy depends on how well and reliably non-noble metal-based electrocatalysts can be made for the oxygen evolution reaction. Here, in the present work, a two-step hydrothermal method was employed to construct 3-dimensional (3D) Mn-doped iron selenide with microsphere architecture. The electrode’s distinctive 3D microsphere-like morphology leads to more active sites and faster electron movement over the perfect electrode, making it easier to release O2 bubbles generated during oxygen evolution reaction (OER) catalysis. As a result, 10% Mn-doped iron selenide outperforms the lower overpotential of (133 mV) at a benchmark current density (j) deposited on the graphite pencil electrode (GPE). Hence, Mn-based electrocatalyst is one of the most intriguing possible applications.

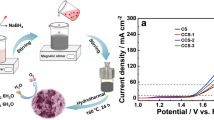
Graphical abstract
Highlights
-
In the present study, 3-dimensional mesoporous Mn-doped iron selenide microspheres morphology was fabricated by employing a two-step hydrothermal route.
-
These remarkable 3-dimensional microsphere structures of 10% Mn-doped iron selenide release the O2 bubbles produced during OER catalysis.
-
The 10% Mn-doped iron selenide exhibits an outstanding OER activity having an ultralow overpotential of 133 mV to achieve a current density of 10 mA/cm2.
-
The 10% Mn-doped iron selenide exhibits the highest stability of 30 h in alkaline solutions.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Xia W, Apergis N, Bashir MF, Ghosh S, Doğan B, Shahzad U (2022) Investigating the role of globalization, and energy consumption for environmental externalities: empirical evidence from developed and developing economies. Renew Energy 183:219–228
Khan MB, Saleem H, Shabbir MS, Huobao X (2022) The effects of globalization, energy consumption and economic growth on carbon dioxide emissions in South Asian countries. Energy Environ 33:107–134
Ehigiamusoe KU, Dogan E (2022) The role of interaction effect between renewable energy consumption and real income in carbon emissions: Evidence from low-income countries. Renew Sustain Energy Rev 154:111883
Mohsin M, Kamran HW, Nawaz MA, Hussain MS, Dahri AS (2021) Assessing the impact of transition from nonrenewable to renewable energy consumption on economic growth-environmental nexus from developing Asian economies. J Environ Manag 284:111999
Kirikkaleli D, Adebayo TS (2021) Do renewable energy consumption and financial development matter for environmental sustainability? New global evidence. Sustain Dev 29:583–594
He W, Abbas Q, Alharthi M, Mohsin M, Hanif I, Vo XV, Taghizadeh-Hesary F (2020) Integration of renewable hydrogen in light-duty vehicle: nexus between energy security and low carbon emission resources. Int J Hydrog Energy 45:27958–27968
Gautam P, Kumar S, Lokhandwala S (2019) Energy-aware intelligence in megacities In Current Developments in Biotechnology and Bioengineering, Elsevier, pp. 211–238
Nisa MU, Manzoor S, Abid AG, Tamam N, Abdullah M, Najam-Ul-Haq M, Al-Buriahi M, Alrowaili Z, Mahmoud ZM, Ashiq MN (2022) CdSe supported SnO2 nanocomposite with strongly hydrophilic surface for enhanced overall water splitting. Fuel 321:124086
Capurso T, Stefanizzi M, Torresi M, Camporeale S (2022) Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers Manag 251:114898
Öberg S, Odenberger M, Johnsson F (2022) Exploring the competitiveness of hydrogen-fueled gas turbines in future energy systems. Int J Hydrog Energy 47:624–644
Yusaf T, Fernandes L, Abu Talib AR, Altarazi YS, Alrefae W, Kadirgama K, Ramasamy D, Jayasuriya A, Brown G, Mamat R (2022) Sustainable aviation—hydrogen is the future. Sustainability 14:548
Singh R, Singh M, Gautam S (2021) Hydrogen economy, energy, and liquid organic carriers for its mobility. Mater Today: Proc 46:5420–5427
Alfryyan N, Manzoor S, Nisa MU et al. (2022) Novel Hydrothermally Synthesized Strontium Telluride Nanoballs as an Efficient Electrocatalyst for Oxygen Evolution Reaction. JOM. https://doi.org/10.1007/s11837-022-05362-5
Manzoor S, Abid AG, Hussain F, Shah A, Pashameah RA, Alzahrani E, El-Bahy SM, Taha T, Ashiq MN (2022) Energy conversion performance of porous ZrTe hybrid derived from chemical transformation of Zr (OH) 4. Fuel 328:125264
Olabi A, Abdelghafar AA, Baroutaji A, Sayed ET, Alami AH, Rezk H, Abdelkareem MA (2021) Large-vscale hydrogen production and storage technologies: Current status and future directions. Int J Hydrog Energy 46:23498–23528
Guo S, Li X, Li J, Wei B (2021) Boosting photocatalytic hydrogen production from water by photothermally induced biphase systems. Nat Commun 12:1–10
Hakeem A, Alshahrani T, Muhammad G, Alhossainy MH, Laref A, Khan AR, Ali I, Farid HMT, Ghrib T, Ejaz SR, Khosa RY, (2021) Magnetic, dielectric and structural properties of spinel ferrites synthesized by sol-gel method. J Mat Res Technol 11:158–169
Javaid R (2021) Catalytic hydrogen production, storage and application. Multidisciplinary Digital Publishing Institute, pp. 836
You B, Sun Y (2018) Innovative strategies for electrocatalytic water splitting. Acc Chem Res 51:1571–1580
Anantharaj S, Ede S, Karthick K, Sankar SS, Sangeetha K, Karthik P, Kundu S (2018) Precision and correctness in the evaluation of electrocatalytic water splitting: revisiting activity parameters with a critical assessment. Energy Environ Sci 11:744–771
Wang S, Lu A, Zhong C-J (2021) Hydrogen production from water electrolysis: role of catalysts. Nano Convergence 8:4
Katubi KM, Nisa MU, Manzoor S, Ahmad Z, Abid AG, Abdullah M, Al-Buriahi MS, Ashiq MN (2022) Hydrothermally fabricated NdTe hollow shells thermally vaporized on Ni foam for water-splitting in alkaline media. Appl Phys A 128:1–10
Alharbi F, Nisa MU, Hassan HMA, Manzoor S, Ahmad Z, Abid AG, Aman S, Ashiq MN, El-Nasser KS, Taha TAM (2022) Novel lanthanum sulfide–decorated zirconia nanohybrid for enhanced electrochemical oxygen evolution reaction. J Solid State Electrochem 26:2171–2182
Shi Q, Zhu C, Du D, Lin Y (2019) Robust noble metal-based electrocatalysts for oxygen evolution reaction. Chem Soc Rev 48:3181–3192
Farid HMT, Ahmad I, Ali I, Ramay SM, Mahmood A, Murtaza G (2017) Dielectric and impedance study of praseodymium substituted Mg-based spinel ferrites. J Magn Magn Mater 434:143–150
Zheng X, Han X, Zhang Y, Wang J, Zhong C, Deng Y, Hu W (2019) Controllable synthesis of nickel sulfide nanocatalysts and their phase-dependent performance for overall water splitting. Nanoscale 11:5646–5654
Farid HMT, Ahmad I, Ali I, Ramay SM, Mahmood A (2019) Study of spinel ferrites with addition of small amount of metallic elements. J Electroceramics 42:57–66
Abid AG, Ashiq MF, Alfryyan N, Manzoor S, Nisa MU, Al-Buriahi M, Alomairy S, Alrowaili Z, Ashiq MN (2022) Stainless steel supported NiS/CeS nanocomposite for significantly enhanced oxygen evolution reaction in alkaline media. J Solid State Electrochem 26:2107–2118
Over H (2021) Fundamental studies of planar single-crystalline oxide model electrodes (RuO2, IrO2) for acidic water splitting, ACS. Catalysis 11:8848–8871
Miao X, Zhang L, Wu L, Hu Z, Shi L, Zhou S (2019) Quadruple perovskite ruthenate as a highly efficient catalyst for acidic water oxidation. Nat Commun 10:1–7
Farid HMT, Mera A, Al-Muhimeed TI, AlObaid AA, Albalawi H, Hegazy HH, Ejaz SR, Khosa RY, Mehmood S, Ali Z (2021) Optoelectronic and thermoelectric properties of A3AsN (A = Mg, Ca, Sr and Ba) in cubic and orthorhombicphase. J Mater Res Technol 13:1485–1495
Zhang L, Wilkinson DP, Liu Y, Zhang J (2018) Progress in nanostructured (Fe or Co)/N/C non-noble metal electrocatalysts for fuel cell oxygen reduction reaction. Electrochim Acta 262:326–336
Liu W, Zhang H, Li C, Wang X, Liu J, Zhang X (2020) Non-noble metal single-atom catalysts prepared by wet chemical method and their applications in electrochemical water splitting. J Energy Chem 47:333–345
Yan L, Zhang B (2021) Aligned Co3O4–CoOOH heterostructure nanosheet arrays grown on carbon paper with oxygen vacancies for enhanced and robust oxygen evolution. Int J Hydrog Energy 46:34287–34297
Ilanchezhiyan P, Kumar GM, Siva C, Madhankumar A, Jeon H, Kang T, Kim D (2019) Evidencing enhanced oxygen and hydrogen evolution reactions using In–Zn–Co ternary transition metal oxide nanostructures: a novel bifunctional electrocatalyst. Int J Hydrog Energy 44:23081–23090
Hu Q, Li G, Han Z, Wang Z, Huang X, Yang H, Zhang Q, Liu J, He C (2019) Recent progress in the hybrids of transition metals/carbon for electrochemical water splitting. J Mater Chem A 7:14380–14390
He T, Matta SK, Will G, Du A (2019) Transition‐metal single atoms anchored on graphdiyne as high‐efficiency electrocatalysts for water splitting and oxygen reduction. Small Methods 3:1800419
Yang C, Lu Y, Duan W, Kong Z, Huang Z, Yang T, Zou Y, Chen R, Wang S (2021) Recent progress and prospective of nickel selenide-based electrocatalysts for water splitting. Energy Fuels 35:14283–14303
Yu J, Cheng G, Luo W (2018) 3D mesoporous rose-like nickel-iron selenide microspheres as advanced electrocatalysts for the oxygen evolution reaction. Nano Res 11:2149–2158
Tao L, Wang Y, Zou Y, Zhang N, Zhang Y, Wu Y, Wang Y, Chen R, Wang S (2020) Charge transfer modulated activity of carbon‐based electrocatalysts. Adv Energy Mater 10:1901227
Swesi AT, Masud J, Liyanage WP, Umapathi S, Bohannan E, Medvedeva J, Nath M (2017) Textured NiSe 2 film: bifunctional electrocatalyst for full water splitting at remarkably low overpotential with high energy efficiency. Sci Rep. 7:1–11
Lin J, Wang H, Yan Y, Cao J, Qu C, Zheng X, Feng J, Qi J (2020) Sandwich-like structured NiSe2/Ni2P@ FeP interface nanosheets with rich defects for efficient electrocatalytic water splitting. J Power Sources 445:227294
Sun H, Yang J-M, Li J-G, Li Z, Ao X, Liu Y-Z, Zhang Y, Li Y, Wang C, Tang J (2020) Synergistic coupling of NiTe nanoarrays with RuO2 and NiFe-LDH layers for high-efficiency electrochemical-/photovoltage-driven overall water splitting. Appl Catal B Environ 272:118988
Pan Y, Zheng F, Wang X, Qin H, Liu E, Sha J, Zhao N, Zhang P, Ma L (2020) Enhanced electrochemical hydrogen evolution performance of WS2 nanosheets by Te doping. J Catal 382:204–211
Mingli F, Xue L, Dandan W, Yinling W, Maoguo L (2019) Fabrication of Te@ NiTe2/NiS heterostructures for electrocatalytic hydrogen evolution reaction. Electrochim Acta 328:135075
Sadaqat M, Manzoor S, Nisar L, Hassan A, Tyagi D, Shah JH, Ashiq MN, Joya KS, Alshahrani T, Najam-ul-Haq M (2021) Iron doped nickel ditelluride hierarchical nanoflakes arrays directly grown on nickel foam as robust electrodes for oxygen evolution reaction. Electrochim Acta 371:137830
Wang G, Huang J, Chen G, Chen W, Song C, Li M, Wang X, Chen D, Zhu H, Zhang X (2020) In-situ-engineered 3D Cu3Se2@ CoSe2–NiSe2 nanostructures for highly efficient electrocatalytic water splitting. ACS Sustain Chem Eng 8:17215–17224
Feng J-X, Wu J-Q, Tong Y-X, Li G-R (2018) Efficient hydrogen evolution on Cu nanodots-decorated Ni3S2 nanotubes by optimizing atomic hydrogen adsorption and desorption. J Am Chem Soc 140:610–617
Shao L, Liang Z-X, Chen H, Song Z-X, Deng X-H, Huo G, Kang X-M, Wang L, Fu X-Z, Luo J-L (2020) CuCo2S4 hollow nanoneedle arrays supported on Ni foam as efficient trifunctional electrocatalysts for overall water splitting and Al–Air batteries. J Alloy Compd 845:155392
Majhi KC, Yadav M (2021) Bimetallic chalcogenide nanocrystallites as efficient electrocatalyst for overall water splitting. J Alloy Compd 852:156736
Zhang W, Liu M, Liang H, Cui L, Yang W, Razal JM, Liu J (2021) Flower-like nanosheets directly grown on Co foil as efficient bifunctional catalysts for overall water splitting. J colloid interface Sci 587:650–660
Ashraf MA, Yang Y, Zhang D, Pham BT (2020) Bifunctional and binder-free S-doped Ni-P nanospheres electrocatalyst fabricated by pulse electrochemical deposition method for overall water splitting. J Colloid Interface Sci 577:265–278
Qu Y, Yang M, Chai J, Tang Z, Shao M, Kwok CT, Yang M, Wang Z, Chua D, Wang S (2017) Facile synthesis of vanadium-doped Ni3S2 nanowire arrays as active electrocatalyst for hydrogen evolution reaction. ACS Appl Mater interfaces 9:5959–5967
Acknowledgements
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project (Grant No. PNURSP2022R70), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alburaih, H.A., Ansari, M.Z., Abid, A.G. et al. Study on active sites of Mn-doped iron selenide on pencil electrode for electrocatalytic water splitting. J Sol-Gel Sci Technol 106, 1–9 (2023). https://doi.org/10.1007/s10971-022-05961-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05961-3