Abstract
MgF2 coating solutions were prepared by the fluorolytic processing of Mg(OEt)2 and MgCl2. Parts of these sols are solvothermally treated in an autoclave at 160 °C. The two respective precursors were used to prepare porous λ/4 antireflective films by dip-coating on soda-lime and borosilicate glass substrates. UV–Vis spectroscopy and X-Ray diffraction (XRD) were applied to characterize the films as a function of annealing temperature. Samples treated at 500 °C underwent testing of abrasion resistance and water solubility.

Porous antireflective MgF2 coatings were prepared from as-synthesized solutions and sols that had additionally undergone solvothermal processing. Films prepared on soda-lime and borosilicate glass substrates were systematically compared.
Highlights
-
Parallel investigation of MgF2 antireflective coatings prepared from as-prepared sols and sols that have been solvothermally treated.
-
Systematic comparison of results obtained on soda-lime and borosilicate glass substrates.
-
Testing of abrasion resistance of porous films.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Already for long, antireflective coatings have gained large commercial attention due to their possible application for optical components, eyeglasses, architectural glazing, and solar panels. Antireflective properties can be achieved by λ/4 single layers, multiple interference films, and so-called moth-eye structures [1]. All these concepts can be realized by sol–gel processing from liquid precursors [2]. Academic feasibility studies can only be a starting point for any practical application, where mechanical and chemical resistance as well as manufacturing costs play a decisive role [3]. In view of their simple single-layer structure, λ/4 layers deserve a great deal of interest in the latter respect.
In λ/4 films, an intermediate refractive index between the substrate and the environment has to be established. For glass substrates and air no dense material fulfills this requirement, therefore, porous films have to be used. Unfortunately, such microstructures are especially susceptible to mechanical damage.
Silica is a material broadly used in sol–gel processing. Due to its refractive index of 1.46, λ/4 layers [3] based on SiO2 need to have a porosity of ~50% for glass substrates. With 1.38 the refractive index of magnesium fluoride is considerably lower. Therefore, MgF2 λ/4 antireflective films only require a porosity of 30% which gives them a higher mechanical stability [3].
Besides routes, where MgF2 is formed from trifluroacetic acid during thermolysis of the films [4], anhydrous coating solutions containing MgF2 particles have been developed [5] and used for the preparation of antireflective films [6]. Even though these systems exhibit remarkable abrasion resistance [3, 7], the material is partially dissolved from glass substrates by water at ambient temperature [8].
It has been shown that the crystallinity of MgF2 particles in solution can be improved by solvothermal treatment in an autoclave at 160 °C [9] and even the coating of thermally labile polymer substrates is possible. It therefore can be assumed that such films on glass may show an improved abrasion resistance and reduced solubility in water. In this paper, we describe the systematic comparison of MgF2 films obtained from as-synthesized MgF2 sols to their solvothermally treated counterparts.
2 Experimental procedure
2.1 Synthesis of coating solutions and film preparation
Dried methanol was obtained from Carl Roth (max. 50 ppm H2O). Magnesium ethoxide Mg(OEt)2 was purchased from STREM Chemicals (97%). MgCl2 was obtained from Sigma Aldrich.
The synthesis of the MgF2 coating solutions is based on a method previously reported [10] and was carried out at the Humboldt University Berlin in the Kemnitz group: MgCl2 was dissolved in methanol before Mg(OEt)2 was added (molar ratio 15:85, overall Mg concentration 0.6 M). This dispersion was stirred for 10 min before methanolic HF (23.3 M) is added slowly under vigorous stirring. The reaction mixture is stirred for at least 1 day.
Parts of this coating solution were solvothermally treated [9] by sealing in PTFE-coated vessels and heating to 160 °C within 0,5 h. After a dwell time of 16 h, the containers were cooled down to ambient temperature within 6 h. The resulting solutions were directly used for coatings without any further treatment.
Film preparation: MgF2 thin films were prepared by dip coating on borosilicate glass (Schott Borofloat®) soda lime glass (Euroglas Germany, Eurofloat®) at the size of 3.3 * 150 * 100 mm. Before the coating experiment, the substrates were cleaned in a laboratory dishwasher by an alkaline cleaning procedure with a final neutralization step. Final curing of films was performed by placing the samples in an oven (Model Thermicon P, Heraeus Instruments, Hanau, Germany) at ambient conditions. Then the temperature was raised to the designated temperature within 2 h. After a dwell time of 10 min the samples were allowed to cool down to room temperature overnight.
2.2 Material characterization
The transmittance and reflectance of films in the spectral range between 300 nm and 2400 nm were determined using a Shimadzu UV-3100 UV–VIS–NIR recording spectrometer combined with a MPC-3100 multi-purpose large sample compartment for UV-3100. The respective film thickness was calculated according to the method described by Diaz [11].
For X-ray diffractometry (XRD) of film grazing incidence X-ray diffractometry (GIXRD; Rigaku SmartLab 3 kW) was performed at an incidence angle of 0.5°. Crystallite sizes were calculated by applying the Scherrer equation to the (111) reflex of MgF2 between 39° and 42°.
Ellipsometric measurements were performed by means of a GES-5E EP-A instrument (Sopra, Paris, France) and also evaluated with the software “WinElli II”. Prior to the measurement, the samples were rinsed with de-ionized water, immersed for 5 min in ethanol, and dried for 5 min at 180 °C in a vented furnace. Open porosity was determined by using the change of the refractive index during water-vapor adsorption and desorption [12, 13]. Based on the Kelvin equation BJH theory was applied to calculate pore radius distributions from the desorption isotherms.
The mechanical stability of the films was tested by a custom-made Crockmeter test using steel wool of the fineness 0000 as abrasive. The stamp (contact area 4.5 cm²) was pressed on the sample with a force of 4 N.
For testing the film solubility samples were partially immersed into an excess of stirred distilled water at room temperature and visually monitored as a function of time.
3 Results and discussion
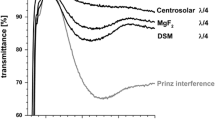
As the optical properties are the key features of AR coatings, MgF2 films prepared on soda-lime and borosilicate glass substrates were characterized as a function of annealing temperature (Fig. 1). Samples treated at 300 °C exhibit a reflectance above 1%. For all higher temperatures values below 1% are observed between 550 and 800 nm. The spectra for films prepared on borosilicate glass are virtually identical, only marginal differences can be seen on soda lime glass as a function of annealing temperature.
The film thicknesses were calculated from the optical spectra [11]. Figure 2 shows that the MgF2 films only undergo minor thermal densification. Whereas the film shrinkage is very steady on borosilicate glass, a steeper decrease of 5 nm is observed for the layers deposited on soda-lime glass between 300 and 350 °C.
Films were inspected by XRD. For both substrate types, first reflexes appear after annealing at 300 °C. Since only patterns of MgF2 become apparent in the 2Θ range between 20° and 70°, the investigation was focused on the (111) main reflex between 39° and 42°. For films prepared on soda-lime glass (Fig. 3a) the peak intensity steadily increases up to an annealing temperature of 450 °C and remains unchanged for films treated at 500 °C. On borosilicate glass, the signals for annealing temperatures between 400 and 500 °C are roughly similar (Fig. 3a).
Even though it is quite difficult to obtain quantitative assessments regarding film crystallinity from XRD data, the reflex width can be used to calculate crystallite sizes using the Scherrer equation. These results are summarized in Fig. 3c.
The grain size of MgF2 films prepared on soda-lime glass generally exceeds the values obtained on borosilicate glass substrates. This observation is in agreement with previous results [6, 8] where it was shown that sodium has a distinct influence on the microstructure of the films. Na ions that either diffuse from the (soda-lime) substrate or are doped to the coating solutions lead to coarser pore structures [8] which is equivalent to larger grain sizes [9].
In Fig. 4 the pore radius distributions as determined by Ellipsometric Porosimetry (EP) are given. The pore sizes generally increase with treatment temperature. The only exception is the samples prepared on soda-lime glass after treatment at 500 °C where the pores are slightly smaller.
For the commercialization of λ/4 layers, their mechanical stability is a decisive factor. Crockmeter testing is a common method to investigate the abrasion resistance of surfaces. Normally, a stamper made of felt is used for rubbing the specimen with a given force. Due to the high durability of our MgF2 films we had to substitute felt with steel wool to achieve a visible effect. In Fig. 5 the results for MgF2 films obtained from as-synthesized coating solutions that have been annealed at 500 °C are summarized. Whereas the coatings prepared on soda-lime glass become more and more damaged as the number of abrasion cycles increases, the films on borosilicate remain virtually intact. Minor damages originate from the uneven surface of the hand-made steel wool stampers.
The superior mechanical durability of the MgF2 films on borosilicate glass goes along with an increased stability against dissolution in water. As can be seen from Fig. 6 these samples only show minor damage after exposure to water for 6 days. In contrast to that, films deposited on soda-lime glass are already changed after 24 h and after 6 days, they are completely removed.
In Fig. 7a the SEM surface image of a film prepared from an as-synthesized coating solution on borosilicate glass is given, the sample had undergone a thermal treatment at 500 °C. The microstructure consists of globular aggregates (diameter ~20 nm) of finer grains. As crystallite sizes of 50 nm were determined by XRD (Fig. 3) the areas of identical crystallite orientation are believed to exceed particle boundaries. The pores determined by EP (Fig. 4) obviously correspond to the inter-particle voids.
It was not possible, though, to determine significant structural differences by SEM imaging between samples as a function of annealing temperature. The same is true for films deposited on soda-lime and borosilicate glass. Cross-sectional views (data not shown) did not reveal any substantial information to elucidate differences in film adhesion and solubility. SEM investigations only map a relatively small sample region and may be distorted by subjective image selection. We, therefore, think that methods such as XRD and EP are more reliable since they average over a large film region.
As-synthesized MgF2 coatings have previously been solvothermally treated in an autoclave. Due to the consolidation of the particles in the solution, it was possible to apply antireflective coatings on thermally labile PMMA substrates without any annealing step [9].
Figure 7b shows the surface pattern of a sample prepared from a solvothermally treated coating solution. The porous film consists of smooth particles with diameters of ~25 nm. In contrast to this, the film prepared from the as-synthesized sol (Fig. 7a) consisted of fine-grained aggregates. Obviously, the solvothermal treatment leads to particle growth in solution. Again it was not possible to detect noteworthy differences induced by thermal treatment or the substrate used.
Now is the question of how such films perform on glass substrates. Our initial guess was that the early MgF2 consolidation should strengthen the film microstructure. Films prepared from solvothermally treated precursors already show antireflective properties after drying at 200 °C (Fig. 8). On soda-lime and borosilicate glass substrates annealing at temperatures above 350 °C leads to identical spectra. The minima of the reflectance in both cases are close to zero and lower than those of films prepared from as-synthesized sols (Fig. 1).
The thicknesses (Fig. 9) as calculated from the optical spectra show that the films undergo a steady shrinkage over the annealing temperatures under investigation.
XRD measurements were performed on the films obtained using solvothermally treated sols (Fig. 10a, b). On both substrate types, distinct reflexes are observed on samples that only have been annealed at 200 °C. The solvothermal treatment at 160 °C obviously already has induced MgF2 crystallization. This result is supported by the SEM results in Fig. 7. The diffraction only shows minor changes at higher temperatures. The analysis of the crystallite sizes (Fig. 10 c) reveals only marginal changes up to 350 °C irrespective of the substrate used. This is a significant difference from the as-synthesized coating solutions, where sodium ions from soda-lime glass substrates had a pronounced impact on the MgF2 crystallization (Fig. 3c).
In Fig. 11 the respective pore radius distributions as determined by EP are given. Similar results are obtained for soda-lime and borosilicate glass substrates. Additionally, it can be seen that at annealing temperatures above 300 °C the films only undergo minor microstructural changes. This is in agreement with the above XRD results were no significant crystallite growth was observed in this temperature region. Compared to films obtained from as-synthesized coating solutions (Fig. 4) the pore sizes are tenentially smaller.
The results from crockmeter testing, however, were very disappointing (Fig. 12): On both glasses, the films (500 °C annealing temperature) are basically completely removed by the first abrasion cycle. No significant difference was observed between the substrate types. Obviously, the partially crystalline MgF2 particles pre-formed during solvothermal treatment only undergo weak bonding to each other and/or to the substrates during annealing. In contrast to that, the films prepared from as-synthesized MgF2 sols showed a good abrasion resistance (Fig. 5). It seems that by the on-substrate crystallization a mechanically much more stable microstructure is established.
The poor mechanical microstructure is also confirmed in water solubility testing of specimen annealed at 500 °C (Fig. 13). MgF2 films prepared from solvothermally treated solutions on soda-lime glass are almost completely removed after one day of water exposure. The samples deposited on borosilicate substrates only exhibit an insignificantly lower solubility.
4 Conclusions
The solvothermal treatment of MgF2 precursors results in the formation of pre-crystallized particles in the solution. Coatings with a minimum reflectance close to zero can be prepared on glass. It previously has been shown that AR coatings on thermally labile polymers are obtainable from such sols [9]. Despite these promising findings, however, these particles undergo limited adhesion to each other and to glass substrates upon thermal treatment. This leads to poor abrasion resistance and instability against detachment in water.
In contrast to this, films with excellent mechanical stability and satisfactory insolubility in water can be prepared from as-synthesized MgF2 coating solutions. In this context coatings prepared on borosilicate glass outperform their counterparts deposited on soda-lime substrates. The crystallite size does not have a significant influence on film stability.
References
Raut HK, Ganesh VA, Nair AS et al. (2011) Anti-reflective coatings: a critical, in-depth review. Energy Environ Sci 4:3779. https://doi.org/10.1039/c1ee01297e
Löbmann P (2013) Antireflective coatings and optical filters. In: Schneller T, Waser R, Kosec M et al., (eds) Chemical solution deposition of functional oxide thin films, vol 29. Springer Vienna, Vienna, p 707–724. s.l
Löbmann P (2017) Antireflective coatings by sol–gel processing: commercial products and future perspectives. J Sol–Gel Sci Technol 83:291–295. https://doi.org/10.1007/s10971-017-4408-x
Shinobu F, Tada M, Kimura T (1997) Preparation and characterization of MgF2 thin film by a trifluoroacetic acid method. Thin Solid Films 304:252–255. https://doi.org/10.1016/S0040-6090(97)00156-9
Scheurell K, Kemnitz E (2018) Fluorolytic sol–gel synthesis of nanometal fluorides: accessing new materials for optical applications. Inorganics 6:128. https://doi.org/10.3390/inorganics6040128
Löbmann P (2018) Sol-gel processing of MgF2 antireflective coatings. Nanomaterials 8. https://doi.org/10.3390/nano8050295
Scheurell K, Kemnitz E, Garcia-Juan P et al. (2015) Porous MgF2 antireflective λ/4 films prepared by sol–gel processing: comparison of synthesis approaches. J Sol-Gel Sci Technol 76:82–89. https://doi.org/10.1007/s10971-015-3754-9
Hegmann J, Jahn R, Löbmann P (2017) Solubility of porous MgF2 films in water: influence of glass substrates. J Sol-Gel Sci Technol 82:40–44. https://doi.org/10.1007/s10971-016-4280-0
Jahn R, Löbmann P (2018) MgF2 films prepared from solvothermally treated precursor solutions. J Sol-Gel Sci Technol 85:514–519. https://doi.org/10.1007/s10971-018-4590-5
Krahl T, Broßke D, Scheurell K et al. (2016) Novel aspects in the chemistry of the non-aqueous fluorolytic sol–gel synthesis of nanoscaled homodisperse MgF2 sols for antireflective coatings. J Mater Chem C 4:1454–1466
Dıaz-Parralejo A, Caruso R, Ortiz AL et al. (2004) Densification and porosity evaluation of ZrO2–3 mol% Y2O3 sol–gel thin films. Thin Solid Films 458:92–97
Baklanov M, Maex K, Green M (2007) Dielectric films for advanced microelectronics. John Wiley & Sons
Löbmann P (2017) Characterization of sol–gel thin films by ellipsometric porosimetry. J Sol-Gel Sci Technol 84:2–15. https://doi.org/10.1007/s10971-017-4473-1
Acknowledgements
This project was funded by the German Federal Ministry of Economics and Technology (grant 03EN1019A). The authors thank the Kemnitz group (Humboldt University Berlin) for the synthesis of the MgF2 coating solution.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Steenhusen, S., Löbmann, P. Solvothermal treatment of MgF2 coating solutions: a comparative study. J Sol-Gel Sci Technol 105, 50–57 (2023). https://doi.org/10.1007/s10971-022-05958-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05958-y