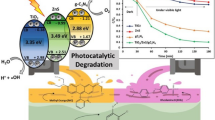
Abstract
In 60 min, microwave-synthesized ZrO2/ZnO heterostructures exhibited high and fast sunlight photodegradation efficiencies for 50 ppm Congo red (CR) and 50 ppm methylene blue (MB) pollutants. ZrO2/ZnO heterostructures were characterized by XRD, SEM, EDX, FTIR, and diffuse reflectance (DR) techniques. The XRD analysis showed that these heterostructures have combined components of tetragonal ZrO2 and hexagonal ZnO phases. The SEM micrographs of all ZrO2/ZnO nanocomposites demonstrate the formation of nanospherical particles (major) and rod-like (minor) structures. The EDX spectra verified the presence of Zr, Zn and O elements with percentage ratios equivalent or close to that used during the experimental preparation. The FT-IR spectra showed the vibrational characteristic absorption modes of ZrO2 and ZnO bonds around 400–600 cm−1. Two band gap energies were estimated corresponding to ZrO2 (5.05–5.16 eV) and ZnO (3.1–3.16 eV) components. Remarkably, in presence of ZrO2/ZnO (30/70 at%) heterostructure, the free solar energy initiated photodegradation efficiencies of 87% and 98% for 50 ppm CR and 50 ppm MB dyes after 60 min, respectively, which indicates the fast and superior photocatalytic activity of microwave-synthesized ZrO2/ZnO heterostructure. As well, this composition reveals good reusability and stability for three photocatalytic cycles. This uppermost photodegradation performance can be assigned to the high separation of charge carriers, especially for the ZrO2/ZnO (30/70 at%) nanocomposite.

Graphical abstract
Highlights
-
Microwave assisted-hydrothermal ZrO2/ZnO heterostructures.
-
ZrO2/ZnO heterostructure exhibits fast and superior sunlight catalytic activity.
-
Efficiency of 87% and 98% for removal of 50 ppm Congo red and methylene blue.
-
High separation of photo-generated electron hole pairs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The elimination of dyes, herbicides, stimulants, pesticides and antibiotics as organic pollutants from the surrounding environment has become a serious world issue in the last years [1,2,3]. Water contaminated with residual dyes from the textile, printing press, paper, cosmetics, leather and other industries is the main source of many environmental problems [4,5,6]. Using solar energy-based semiconductor oxides in photocatalytic degradation of these types of organic pollutants seems to be a promising cost technique [7,8,9]. The light induces heterogeneous catalysis enables the conversion of organic pollutants such as dyes to molecules with higher biodegradability, finally leading to nonhazardous molecules (CO2 and H2O) [10, 11]. As a result, the search for advanced solar energy photo-reactive materials is of great importance environmentally and economically [12, 13]. In studies relating to the photocatalytic degradation of organic dyes pollutants, zinc oxide (ZnO) nanostructures have been extensively candidates as a photocatalysts, owing to their high activity, long life span, low cost and its environmentally friendly nature [14,15,16]. However, the high recombination rate of the photogenerated charge carriers (electrons and holes pairs) acts as a limiting factor restricting the widespread applications of ZnO in photocatalysis [17, 18]. Recently, the ZnO based nanocomposites received a great attention of addressing these obstacles in an operative manner [19, 20]. To clarify, both of the metal oxides semiconductors were instantaneously excited and the electrons slip from the conduction band (CB) of one semiconductor to another while the holes moved to the valence band (VB) in the opposite direction [21,22,23]. These reactions have the ability to separate the electron–hole pairs which in turn enhanced the photocatalytic efficiency [21,22,23]. One of the metal oxide semiconductor materials that improve the photocatalytic properties of ZnO nanoparticles is zirconium oxide (ZrO2) [24,25,26]. ZrO2 belongs to a group of metal oxide semiconductor materials with relatively wide band gap energy over 5 eV [27]. In the past decade, the global research interest in wide band gap semiconductors has been significantly focused on ZrO2/ZnO oxides due to their excellent properties as semiconductor materials [24,25,26]. The high electron mobility, high thermal conductivity, wide and direct band gap, good transparency, large exciton binding energy and easiness of synthesizing make ZrO2/ZnO structure appropriate for a wide range of applications in optoelectronics, transparent electronics, lasing and sensing [28,29,30]. The replacement of Zn2+ by Zr4+ or versa vise can disturbs the charge balance in their lattices and the disturbed charge balance contributes to the adsorption of more hydroxide ions (–OH) on the surface of the particles of the powder, which additionally inhibits the recombination of the electron–hole pairs [21]. Congo red (CR) and methylene blue (MB) dyes have aromatic rings in their structures that are resistant to biodegradation and aerobic degradation. Therefore, it is essential to find operative methods that are useful for environmental protection and human health to remove these dyes from wastewater. In our previous study [21], we found that ZrO2/ZnO (50:50) nanocomposite prepared by sol–gel method possesses degradation efficiencies of 99% and 97% for indigo carmine (IC) (5 × 10−5 M) and MB (2.5 × 10−5 M) in 300 and 150 min, respectively. In this study, we made use of the major rewards of the microwave-activation hydrothermal technique to obtain nano-sized ZrO2/ZnO heterostructures. These advantages include high purity synthesis circumstances, wide range of heating, perfect control of time, and the accessibility to achieve highly active powders with a narrow particle-size distribution. The effect of variation of Zr/Zn atomic ratio on the physicochemical and photocatalytic properties of the synthesized composites was evaluated. The synthesized ZrO2/ZnO heterostructures were used as photocatalysts in the heterogeneous photodegradation of 50 ppm CR and 50 ppm MB dyes. Remarkably, the obtained results revealed that ZrO2/ZnO heterostructure with composition of 30/70 at% has the highest ability to degrade the high concentrations of CR (50 ppm) and MB (50 ppm) in a short time of 60 min compared to ZrO2/ZnO (50:50%) nanocomposite prepared by sol–gel method (300 and 150 min).
2 Experimental: synthesis, characterization and measurements
2.1 Materials and synthesis
Throughout the entire preparation part of this work, double distilled water, as well as the following reagent grade chemicals, were used: zinc acetate dihydrate Zn(CH3COO)2·2H2O, zirconium hydroxide Zr(OH)4, ethylene glycol and nitric acid (HNO3) from Merck, Germany. ZrO2/ZnO nanocomposites were attained through the addition of calculated amounts of zirconium salt (dissolved in 10 ml of 4 N HNO3) to 50 ml aqueous solution of Zn(CH3COO)2·2H2O at the desired atomic percentage (at%) of ZrO2 = 10, 20, 30, and 40 at%. The mixture was stirred well, after that, 5 ml of ethylene glycol (complexing agent) was added drop by drop to stimulate the gelation process. The pH of the solutions was neutralized to 7 by slow addition of aqueous ammonia solution which speeds up the hydrolysis process and initiates the gelation process. After that, the mixture was poured into the Teflon vessel of the microwave reactor (MW) from Plazmatronika Ltd (Warsaw, Poland). The system runs at (600 W, 2.45 GHz, ERTEC microwave reactor). The duration of the reaction was 20 min, temperature 220 °C and power 100%. After the reaction was completed, the reaction vessel was cooled down for 20 min. The obtained powders were sedimented, separated from the solution by filtering and were washed with distilled water and isopropanol.
2.2 Characterization and photocatalytic properties measurements
The physical structure, lattice parameter and crystallite size of ZrO2/ZnO heterostructures with ZrO2 content of 10, 20, 30 and 40 at% were estimated by X-ray diffraction (XRD) with Cu Kα radiation of wavelength λ = 1.5406 Å (XRD, X’Pert PRO diffractometer, PANalytical BV, Almelo, Netherlands) and Rietveld refinement analysis. The morphological and elemental compositions of the synthesized ZrO2/ZnO heterostructures were investigated by the scanning electron microscope (Ultra Plus; Carl Zeiss Meditec AG, Jena, Germany) and energy-dispersive X-ray (EDX) technique. The vibrational absorption modes (Fourier transform infrared (FTIR) spectra) of ZrO2/ZnO heterostructures were detected through using a FTIR spectrometer (JASCO, model 4600). The absorption of wavelengths and the band gap energy were investigated using a double beam spectrophotometer-JASCO (model V-570 UV-Vis-NIR). At room temperature, the photodegradation rate of ZrO2/ZnO heterostructures was tested for CR and MB pollutants under direct free solar irradiation. In each experiment, the photocatalytic test was done by using CR solution with concentration of 50 ppm, MB solution of 50 ppm and 0.05 g of ZrO2/ZnO heterostructures. After mixing the ZrO2/ZnO heterostructure with CR (50 ppm, 100 ml) or MB (50 ppm, 100 ml), the mixtures were stirred for 20 min under dark condition. After this step, the mixed solution (ZrO2/ZnO heterostructure and dye) was placed under direct sunlight irradiation for 60 min. In total, 4 ml of the irradiated solution was withdrawn to measure the effect of time on dye decomposition. After removing the catalyst, the photocatalytic activity (%) was calculated by measuring the absorbance of the analytical samples using Schimadzu UV 3100, JP spectrophotometer.
3 Results and discussion
3.1 XRD study
Figure 1 depicts the XRD patterns of ZrO2/ZnO heterostructures with ZrO2 content of 10, 20, 30 and 40 at% prepared by hydrothermal method. The observed diffraction peaks of the microwave-synthesized ZrO2/ZnO powders were mainly indexed to wurtzite hexagonal structure of ZnO (JCPDS card No. 36-1451, space group P63mc) and tetragonal ZrO2 structure (JCPDS no. 79-1771, space group P42/nmc), signifying the formation of ZrO2/ZnO nanocomposites. In these patterns, the diffraction peaks located at nearly 2θ = 31.86°, 34.48°, 36.31°, 47.64°, 56.93°, 62.94°, 66.45°, 68.01°, 69.15°, 72.73° and 77.02° were related to (100), (002), (101), (102), (110), (103), (200), (112), (201), (004) and (202) crystallographic planes of wurtzite hexagonal ZnO while the diffraction peaks situated at 2θ = 30.85°, 50.08° and 60.02° were assigned to (111), (200) and (313) planes of tetragonal ZrO2. The intensities of the diffraction peaks of the tetragonal phase were increased with increasing the ZrO2 content from 10 to 40 at% (Fig. 1). No observable diffraction peaks related to any secondary phases or any impurities were detected in the patterns. The crystallite sizes (D) of ZrO2/ZnO heterostructures were calculated based on Scherrer equation [30]:
where λ is the X-ray wavelength, θ is the Bragg diffraction angle, 0.89 is Scherrer’s constant and β is the full width at half maximum of the diffraction peaks. The calculated approximate values of the phase ratio, lattice parameters (a, b, c) and unit cell volume (V) of ZrO2 and ZnO components in ZrO2/ZnO heterostructures were determined by least-square Rietveld refinement based on FullProf software [31]. For all ZrO2/ZnO heterostructures, the profiles of the refinement data showed a well-fitting between the experimental and the calculated data, as represented in Fig. 2. Besides, no indication for any chemical products due to the reaction between ZrO2 and ZnO were detected and only the patterns confirmed the nanocomposites formation. As represented in Table 1, the measured phase ratio of ZrO2 is 8.5%, 18.8%, 31.2% and 36.8% for the nanocomposites with desired ZrO2 content of 10, 20, 30 and 40 at%, respectively, which in proximate values with that used in the preparation step. Based on the ionic radii database, the ionic radius of Zn2+ ion is 0.74 Å while that of Zr4+ is 0.72 Å with a clear difference only in the oxidation state. Consequently, it is anticipated that some of the Zr4+ ions can replace the Zn2+ ions into ZnO lattice or versa vise. Due to the difference in charge, Zn2+ cations can be acted as an acceptor dopant for Zr4+ sites and lead to oxygen vacancies formation while Zr4+ ions can act as donor dopants in ZnO lattice which injects more electrons. From the results in Table 1, there are some variations in the lattice parameter (a, b, c) and unit cell volume (V) of ZnO and ZrO2 due to the change in ZrO2 content in ZrO2/ZnO nanocomposites. The average crystallite size of the different ZrO2/ZnO heterostructures was calculated to be 45–51 nm, which confirms the formation of small nano-sized composites. Figure 3 illustrates the spacing-filling model and polyhedral crystal structure of hexagonal ZnO and tetragonal ZrO2.
3.2 SEM-EDX analysis: morphological and compositions
Figure 4 illustrates the scanning electron micrographs and the corresponding 3D view of ZrO2/ZnO heterostructures with ZrO2 content of 10, 20, 30 and 40 at% prepared by microwave-assisted hydrothermal method. The SEM micrograph of ZrO2/ZnO heterostructure with ZrO2 content of 10 at% shows the presence of two types of particles. The major type of these particles has a spherical shape while the minor type possesses an elongated shape, rod-like structure as shown inset Fig. 4a. When the content of ZrO2 reached 20 at%, more fine spherical nanoparticles are observed in addition to the rod-like structure. In the case of 30 at% ZrO2 similar architecture to that of 20 at% ZrO2 was formed. The micrograph of the ZrO2/ZnO heterostructure with ZrO2 content of 40 at% shows more uniform elongated particles in contact with very fine spherical nanoparticles. Focus shot on gathering of spherical nanoparticles (inset Fig. 4d) illustrates that the particles are homogenous and have a nearly similar size. The SEM micrographs of the synthesized ZrO2/ZnO heterostructures clearly show the enhancements in the size and homogeneity for the formed particles with increasing ZrO2 content. Elemental composition of ZrO2/ZnO heterostructures with Zr content from 10 to 40 at% were performed by EDX spectroscopy as shown in Fig. 5. Obviously, the characteristic peaks which corresponding to Zn, Zr and O elements were detected without any sign for the presence of any other impurities elements. With increasing the Zr content, the atomic percent (at%) of the Zn, Zr and O elements (inset Fig. 5) show gradual decreases for Zn element with steady increase for Zr element. The measured percentages of Zn, Zr and O elements in the different ZrO2/ZnO heterostructures are close to the experimental values and it is accepted within the normal error of the EDX technique.
3.3 FT-IR spectra
Figure 6 demonstrates the FTIR spectra of ZrO2/ZnO heterostructures with ZrO2 content of 10, 20, 30 and 40 at% synthesized by microwave-assisted hydrothermal route. Herein, the patterns show two vibrational absorption bands situated around 3428–3432 cm−1 and 1624–1627 cm−1 and both can be ascribed to the stretching and bending vibrational modes of the adsorbed H-O-H molecules on the surface of the ZrO2/ZnO heterostructures, respectively. The characteristic FT-IR vibrational absorption bands in the region of 525–409 cm−1 are attributed to the vibration modes of Zr-O and Zn-O bonds which confirm the formation of ZrO2/ZnO heterostructures. It can be seen that the intensities of these characteristic absorption bands were reduced with increasing the ZrO2 content in the ZrO2/ZnO heterostructures. Besides, the shape of the bands become broader and extends over wide wavenumbers (cm−1). The gradual effect of ZrO2 on the IR properties is in agreement with the increase of ZrO2 content noticed by the XRD analysis.
3.4 Optical properties
Figure 7 illustrates the ultraviolet–visible diffuse reflectance spectra of ZrO2/ZnO heterostructures with ZrO2 content of 10, 20, 30 and 40 at% synthesized by microwave-assisted hydrothermal technique. For all ZrO2/ZnO heterostructures, the reflectance spectra exhibit two absorption lines (sharp decreases in intensity) below 400 and 250 nm which correlated to the band gap energies of ZnO and ZrO2, respectively. It can be observed that the length of the absorption line (sharp decreasing in intensity) of ZnO was reduced with increasing ZrO2 content while the length of the absorption line (intensity) of ZrO2 was increased. The Kubelka–Munk equation, F (R) = (1 − R)2/2R = α/S, was used to estimate the band gap energy of the two components in ZrO2/ZnO heterostructures (i.e., ZrO2 and ZnO) [32]. The relation between hν (eV, X-axis) and [F(R)hν]2 (Y-axis) gives the band gap energy by lengthening the linear part of the curve to cut [F(R)hν]2 = 0, as represented in Fig. 8 and tabulated in Table 1. The band gap energy of ZnO was found to be 3.1, 3.1, 3.15 and 3.18 eV, while the band gap energy of ZrO2 was estimated to be 5.1, 5.05, 5.16 and 5.16 eV for ZrO2/ZnO heterostructures with ZrO2 content of 10, 20, 30 and 40 at%, respectively.
3.5 Photocatalytic properties under sunlight
Under free solar energy irradiation, the photodegradation efficiencies of ZrO2/ZnO heterostructures with ZrO2 content of 10, 20, 30 and 40 at% were assessed for decomposition of anionic CR (50 ppm, 100 ml) and cationic MB (50 ppm, 100 ml). Figures 9 and 10 demonstrate the variations of the maximum absorption peak of CR which located at 497 nm and that of MB situated at 668 nm in the presence of ZrO2/ZnO (30/70 at%) heterostructure after exposed to sunlight irradiation of 60 min. For ZrO2/ZnO (30/70 at%) heterostructure, obvious changes were detected in the maximum absorption peak of the CR (497 nm) and MB (668 nm) dyes after irradiation by sunlight with nearly complete vanishing of these peaks after 60 min. In case of ZrO2/ZnO heterostructure with ZrO2 content of 10, 20 and 40 at% (figures not included here) the decreasing in the maximum absorption peaks of both dyes is still good but less compared to ZrO2/ZnO heterostructure of 30 at% ZrO2 content. Figure 11 illustrates the whole photocatalytic efficiency of ZrO2/ZnO heterostructures for CR (50 ppm) and MB (50 ppm) dyes after 60 min of sunlight radiation. For CR, the photodegradation efficiencies were estimated to be 52%, 70%, 87% and 74% for ZrO2/ZnO heterostructures with ZrO2 content of 10, 20, 30 and 40 at%, respectively. In case of MB, total efficiencies of 56%, 75%, 98% and 76% were detected for these catalysts, respectively. Remarkably, these results confirm that ZrO2/ZnO heterostructure of 30 at% content possesses the highest efficiency for degradation of 50 ppm of both dyes in 60 min only. The observed enhancements in the photocatalytic activity of ZnO nanoparticles upon addition of 30 at% ZrO2 signifying that this composition possesses more efficient for separation of the photogenerated charge carriers (electron–hole pairs). The obtained results revealed that ZrO2/ZnO (30/70 at%) heterostructure synthesized by microwave-assisted hydrothermal method has high activity and fast degradation time (60 min) for organic dyes in comparison with our previous study reporting degradation of MB and IC dyes using sol–gel-assisted ZrO2/ZnO (50:50%) nanocomposite in 300 and 150 min, respectively [21]. The mechanism for organic pollutant dyes degradation (CR and MB) in the presence of ZrO2/ZnO heterostructure was linked to the excitation of electrons by the solar photon energy (Fig. 12). After placed the mixed solution under direct solar energy, the electrons in the VB of ZrO2 and ZnO powders are transferred to their CB, generating electron–hole pairs. In solution, the excited electrons interact with the surround oxygen molecules (O2) to create superoxide radicals (O2˙ˉ) as reactive species along with hydrogen peroxide (H2O2). In a another process, the positive charge (holes) which formed in the VB react with water (H2O) molecules and the hydroxyl species (–OH) to produce hydroxyl radicals (OH˙) as reactive species. Between ZrO2 and ZnO components, the electrons can transfer from the CB of ZrO2 to the CB of ZnO while the holes (h+) in the VB of ZnO can transfer to the VB of ZrO2 which advance the electrons-hole pair’s separation. Efficiently, the hydroxyl radicals (OH˙) and the superoxide radicals (O2˙−) hit the CR and MB molecules in the dirty solution and finally decompose them to non-toxic molecules (H2O and CO2). The total steps of the photocatalytic mechanism can be illuminated through the following chemical reaction [33,34,35,36,37,38]:
In this work, the obtained results show that the best composition for photocatalysis is ZrO2/ZnO heterostructure with content of 30/70 at% and also revealed that the microwave-assisted hydrothermal technique is more effective for the photocatalytic applications of ZrO2/ZnO heterostructure compared to sol–gel method used on our previous study on the same nanocomposite [21]. The microwave-assisted hydrothermal technique helps in reducing the photodegradation to 60 min compared to 150 and 300 min with maintaining the same efficiency and also perfect for high concentrations of organic pollutants [21]. The stability and reusability of ZrO2/ZnO (30/70 at%) heterostructure (high efficient catalyst) was studied for the decomposition of MB dye at similar reaction circumstances. After the first photocatalytic experiment the catalyst was collected, washed with deionized water and dried at 90 °C for 1 h in air atmosphere to be used for the new degradation test. Figure 13 shows the results of MB degradation for three cycles. ZrO2/ZnO (30/70 at%) photocatalyst reveals a good photo-stability for MB decomposition with efficiency of 98%, 92% and 85% for the first, second and third test under solar irradiation for 60 min, respectively. The obtained results point out that ZrO2/ZnO (30/70 at%) photocatalyst is satisfactorily stable during the photo-decomposition of methylene dye.
4 Conclusion
ZrO2/ZnO heterostructure (30/70 at%) exhibited a superior and fast sunlight photodegradation efficiency for high concentrations of CR (50 ppm) and MB (50 ppm) dyes. The XRD and Rietveld refinement results confirm the existence of two sets of diffraction peaks corresponding to tetragonal ZrO2 and hexagonal ZnO structures. The morphological study of ZrO2/ZnO heterostructures demonstrates the formation of a mixture of nanospherical particles (major) and rod-like (minor) structures in each composite. The EDX analysis shows the occurrence of Zr, Zn and O elements with a percentage ratio equivalent or closer to that used in the experimental preparation. Two band gap energies were estimated in each composite corresponding to ZrO2 (5.05–5.16 eV) and ZnO (3.1–3.16 eV) components. The solar energy photodegradation efficiency of ZrO2/ZnO (30/70 at%) heterostructure for 50 ppm CR and 50 ppm MB were found to be 87% and 98% after 60 min of solar energy irradiation, respectively, confirming the higher photocatalytic activity of ZrO2/ZnO heterostructure. ZrO2/ZnO (30/70 at%) heterostructure reveals good reusability and stability for three photocatalytic cycles. The significant photodegradation performance can be correlated to the high separation of charge carriers, especially for ZrO2/ZnO (30/70 at%) heterostructure.
References
Castro LV, Ortíz-Islas E, Manríquez ME, Albiter E, Cabrera-Sierra R, Alvarado-Zavala B (2021) Photocatalytic degradation of mixed dyes in aqueous phase by MgAlTi and ZnAlTi mixed oxides. Top Catal 64:97–111
Smrithi SP, Kottam N, Vergis BR (2022) Heteroatom modified hybrid carbon quantum dots derived from Cucurbita pepo for the visible light driven photocatalytic dye degradation. Top Catal. https://doi.org/10.1007/s11244-022-01581-x
Solís-Casados DA, Martínez-Peña J, Hernández-López S, Escobar-Alarcón L (2020) Photocatalytic degradation of the malachite green dye with simulated solar light using TiO2 modified with Sn and Eu. Top Catal 63:564–574
Franco P, Sacco O, De Marco I, Sannino D, Vaiano V (2020) Photocatalytic degradation of Eriochrome Black-T azo dye using Eu-doped ZnO prepared by supercritical antisolvent precipitation route: a preliminary investigation. Top Catal 63:1193–1205
Chimupala Y, Phromma C, Yimklan S, Semakul N, Ruankham P (2020) Dye wastewater treatment enabled by piezoenhanced photocatalysis of single-component ZnO nanoparticles. RSC Adv 10:28567–28575
Ajmal A, Majeed I, Malik RN, Idriss H, Nadeem MA (2014) Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: a comparative overview. RSC Adv 4:37003–37026
Bora LV, Mewada RK (2017) Photocatalytic treatment of dye wastewater and parametric study using a novel Z-scheme Ag2CO3/SiC photocatalyst under natural sunlight. J Environ Chem Eng 5:5556–5565
Sharma P, Singh MK, Mehata MS (2022) Sunlight-driven MoS2 nanosheets mediated degradation of dye (crystal violet) for wastewater treatment. J Mol Struct 1249:131651
Singh J, Soni RK (2021) Fabrication of nanostructured In2S3 thin film with broad optical absorption for improved sunlight mediated photocatalysis application. Optical Mater 122:111748
Reza KM, Kurny A, Gulshan F (2017) Parameters affecting the photocatalytic degradation of dyes using TiO2: a review. Appl Water Sci 7:1569–1578
Liu T, Wang L, Lu X, Fan J, Cai X, Gao B, Miao R, Wang J, Lv Y (2017) Comparative study of the photocatalytic performance for the degradation of different dyes by ZnIn2S4: adsorption, active species, and pathways. RSC Adv 7:12292–12300
Kubacka A, Caudillo-Flores U, Barba-Nieto I, Fernández-García M (2021) Towards full-spectrum photocatalysis: successful approaches and materials. Appl Catal A Gen 610:117966
Song Y, Zhang J, Dong X, Li H (2021) A Review and recent developments in full-spectrum photocatalysis using ZnIn2S4-based photocatalysts. Energy Technol 9:2100033
Raza W, Faisal SM, Owais M, Bahnemann D, Muneer M (2016) Facile fabrication of highly efficient modified ZnO photocatalyst with enhanced photocatalytic, antibacterial and anticancer activity. RSC Adv 6:78335–78350
El Golli A, Fendrich M, Bazzanella N, Dridi C, Miotello A, Orlandi M (2021) Wastewater remediation with ZnO photocatalysts: green synthesis and solar concentration as an economically and environmentally viable route to application. J Environ Manag 286:112226
Długosz O, Szostak K, Banach M (2020) Photocatalytic properties of zirconium oxide–zinc oxide nanoparticles synthesised using microwave irradiation. Appl Nanosci 10:941–954
Mahardika T, Putri NA, Putri AE, Fauzia V, Roza L, Sugihartono I, Herbani Y (2019) Rapid and low temperature synthesis of Ag nanoparticles on the ZnO nanorods for photocatalytic activity improvement. Results Phys 13:102209
Han C, Yang M, Weng B, Xu Y (2014) Improving the photocatalytic activity and anti-photocorrosion of semiconductor ZnO by coupling with versatile carbon. Phys Chem Chem Phys 16:16891–16903
Kalisamy P, Lallimathi M, Suryamathi M, Palanivel B, Venkatachalam M (2020) ZnO-embedded S-doped g-C3N4 heterojunction: mediator-free Z-scheme mechanism for enhanced charge separation and photocatalytic degradation. RSC Adv 10:28365–28375
Chen C, Bi W, Xia Z, Yuan W, Li L (2020) Hydrothermal synthesis of the CuWO4/ZnO composites with enhanced photocatalytic performance. ACS Omega 5:13185–13195
Wahba MA, Yakout SM, Mohamed WAA, Galal HR (2020) Remarkable photocatalytic activity of Zr doped ZnO and ZrO2/ZnO nanocomposites: structural, morphological and photoluminescence properties. Mater Chem Phys 256:123754
Zouhier M, Tanji K, Navio JA, Hidalgo MC, Jaramillo-Paez C, Kherbeche A (2020) Preparation of ZnFe2O4/ZnO composite: effect of operational parameters for photocatalytic degradation of dyes under UV and visible illumination. J Photochem Photobiol A Chem 390:112305
Saravanan R, Karthikeyan S, Gupta VK, Sekaran G, Narayanan V, Stephen A (2013) Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater Sci Eng C 33:91–98
Aghabeygi S, Khademi-Shamami M (2018) ZnO/ZrO2 nanocomposite: sonosynthesis, characterization and its application for wastewater treatment. Ultrason Sonochem 41:458–465
Gurushantha K, Renuka L, Anantharaju KS, Vidya YS, Nagaswarupa HP, Prashantha SC, Nagabhushana H (2017) Photocatalytic and photoluminescence studies of ZrO2/ZnO nanocomposite for LED and Waste water treatment applications. Mater Today Proc 4:11747–11755
López MCU, Lemus MAA, Hidalgo MC, González RL, Owen PQ, Oros-Ruiz S, López SAU, Acosta J (2019) Synthesis and characterization of ZnO-ZrO2 nanocomposites for photocatalytic degradation and mineralization of phenol. J Nanomater 2019:1015876
Wang Q, Edalati K, Koganemaru Y, Nakamura S, Watanabe M, Ishihara T, Horita Z (2020) Photocatalytic hydrogen generation on low band gap black zirconia (ZrO2) produced by high pressure torsion. J Mater Chem A 8:3643–3650
Ariza R, Dael M, Sotillo B, Urbieta A, Solis J, Fernández P (2021) Vapor-solid growth ZnO:ZrO2 micro and nanocomposites. J Alloy Compd 877:160219
Qadri SB, Horwitz JS, Chrisey DB (2000) Transparent conducting films of ZnO–ZrO2: structure and properties. J Appl Phys 88:6564
Velumani M, Meher SR, Balakrishnan L, Sivacoumar R, Alex ZC (2016) ZrO2-ZnO composite thin films for humidity sensing. AIP Conf Proc 1731:080032
Chanda A, Gupta S, Vasundhara M, Joshi SR, Mutta GR, Singh J (2017) Study of structural, optical and magnetic properties of cobalt doped ZnO nanorods. RSC Adv 7:50527–50536
Baqiah H, Talib ZA, Shaari AH, Dihom MM, Kechik MMA, Chen SK, Liew JYC, Zainal Z, Fudzi LM (2019) Structural, optical, magnetic and photoelectrochemical properties of (BiFeO3)1−x(Fe3O4)x nanocomposites. J Sol-Gel Sci Technol 91:624–633
Tama AM, Das S, Dutta S, Bhuyan MDI, Islam MN, Basith MA (2019) MoS2 nanosheet incorporated α-Fe2O3/ZnO nanocomposite with enhanced photocatalytic dye degradation and hydrogen production ability. RSC Adv 9:40357–40367
Abebe B, Zereffa EA, Murthy HCA (2021) Synthesis of poly(vinyl alcohol)-aided ZnO/Mn2O3 nanocomposites for acid orange-8 dye degradation: mechanism and antibacterial activity. ACS Omega 6:954–964
Fang H, Guo Y, Wu T, Liu Y (2018) Biomimetic synthesis of urchin-like CuO/ZnO nanocomposites with excellent photocatalytic activity. N J Chem 42:12779–12786
Kumaresan N, Maria M, Sinthiya A, Ramamurthi K, Babu RR, Sethuraman K (2020) Visible light driven photocatalytic activity of ZnO/CuO nanocomposites coupled with rGO heterostructures synthesized by solid-state method for RhB dye degradation. Arab J Chem 13:3910–3928
Hayati F, Isari AA, Fattahi M, Anvaripour B, Jorf S (2018) Photocatalytic decontamination of phenol and petrochemical wastewater through ZnO/TiO2 decorated on reduced graphene oxide nanocomposite: influential operating factors, mechanism, and electrical energy consumption. RSC Adv 8:40035–40053
Kumar A, Rout L, Achary LSK, Mohanty A, Marpally J, Chand PK, Dash P (2016) Design of binary SnO2-CuO nanocomposite for efficient photocatalytic degradation of malachite green dye. AIP Conf Proc 1724:020027
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wahba, M.A., Yakout, S.M. Microwave-synthesized ZrO2/ZnO heterostructures: fast and high charge separation solar catalysts for dyes-waste degradation. J Sol-Gel Sci Technol 104, 330–341 (2022). https://doi.org/10.1007/s10971-022-05936-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05936-4