Abstract
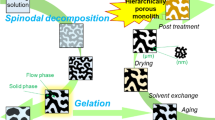
Cosolvent-free (solventless) acid-catalyzed partial hydrolysis of tetramethoxysilane (TMOS) or tetraethoxysilane (TEOS) followed by neutralization with imidazole (pKa = 7.0) was employed to form macroporous silica gels via polycondensation-induced phase separation and concurrent gelation. The reactions of TEOS at 20 °C and subsequent drying in air yielded crack-free opaque macroporous xerogels. In contrast, cooling was necessary to derive opaque gels from TMOS, and the resulting xerogels obtained by reactions at 5 °C broke into pieces during drying due to the too small size of the pores. A macroporous silica xerogel prepared from 50 mmol (10.4 g) of TEOS was dried within 30 h in air, and subsequent sintering in a helium atmosphere at 1300 °C yielded a monolithic silica glass. The yield of silica glasses was higher than 99%.

Photographs of two opaque macroporous xerogels prepared by cosolvent-free acid-catalyzed partial hydrolysis of TEOS followed by neutralization-induced gelation in parallel with phase separation, and silica glasses derived from these gels. The amount of TEOS used was 50 mmol for the gel and glass in the left column, and 100 mmol for those in the center and right columns.
Highlights
-
Formation of monolithic macroporous silica gels was examined by a cosolvent-free (solventless) sol–gel method using tetraethoxysilane (TEOS) or tetramethoxysilane (TMOS) as silicon sources.
-
Acid-catalyzed partial hydrolysis of TEOS and subsequent neutralization with imidazole (pKa = 7.0) at 20 °C caused gelation accompanied by phase separation and formed opaque macroporous silica gels, which were easily dried in air without fracture and sintered into monolithic silica glasses with high yields exceeding 99%.
-
Cooling was necessary to form opaque gels from TMOS, whereas the resulting xerogels were fractured because of the too small size of the pores.




Similar content being viewed by others
References
Dislich H (1971) New routes to multicomponent oxide glasses. Angew Chem Int Ed 10:363–370. https://doi.org/10.1002/anie.197103631
Yamane M, Aso S, Okano S, Sakaino T (1979) Low temperature synthesis of a monolithic silica glass by the pyrolysis of a silica gel. J Mater Sci 14:607–611. https://doi.org/10.1007/BF00772720
Yoldas BE (1979) Monolithic glass formation by chemical polymerization. J Mater Sci 14:1843–1849. https://doi.org/10.1007/BF00551023
Nogami M, Moriya Y (1980) Glass formation through hydrolysis of Si(OC2H5)4 with NH4OH and HCl solution. J Non-Cryst Solids 37:191–201. https://doi.org/10.1016/0022-3093(80)90150-7
Sakka S, Kamiya K (1980) Glasses from metal alcoholates. J Non-Cryst Solids 42:403–422. https://doi.org/10.1016/0022-3093(80)90040-X
Mukherjee SP (1980) Sol–gel processes in glass science and technology. J Non-Cryst Solids 42:477–488. https://doi.org/10.1016/0022-3093(80)90046-0
Ulrich DR (1988) Prospects of sol–gel processes. J Non-Cryst Solids 100:174–193. https://doi.org/10.1016/0022-3093(88)90015-4
Zarzycki J, Prassas M, Phalippou J (1982) Synthesis of glasses from gels: the problem of monolithic gels. J Mater Sci 17:3371–3379. https://doi.org/10.1007/BF01203507
Kistler SS (1931) Coherent expanded aerogels and jellies. Nature 127:741. https://doi.org/10.1038/127741a0
Rabinovich EM, Johnson DW, MacChesney JB, Vogel EM (1982) Preparation of transparent high-silica glass articles from colloidal gels. J Non-Cryst Solids 47:435–439. https://doi.org/10.1016/0022-3093(82)90221-6
Toki M, Miyashita S, Takeuchi T, Kanbe S, Kochi A (1988) A large-size silica glass produced by a new sol–gel process. J Non-Cryst Solids 100:479–482. https://doi.org/10.1016/0022-3093(88)90067-1
Fujimoto Y, Yoshida H, Nakatsuka M, Ueda T, Fujinoki A (2005) Development of Nd-doped optical gain material based on silica glass with high thermal shock parameter for high-average-power laser. Jpn J Appl Phys 44:1764–1770. https://doi.org/10.1143/JJAP.44.1764
Yamane M, Okano S (1979) Low temperature synthesis of a monolithic silica glass. J Ceram Soc Jpn (Yogyo-Kyokai-Shi) 87:434–438. https://doi.org/10.2109/jcersj1950.87.1008_434
Kawaguchi T, Hishikura H, Iura J, Kokubu Y (1984) Monolithic dried gels and silica glass prepared by the sol–gel process. J Non-Cryst Solids 63:61–69. https://doi.org/10.1016/0022-3093(84)90386-7
Matsuyama I, Susa K, Satoh S, Goo JK (1992) Foaming phenomena in sol–gel-derived glasses. J Non-Cryst Solids 151:160–168. https://doi.org/10.1016/0022-3093(92)90024-E
Kirkbir F, Murata H, Meyers D, Chaudhuri SR, Sarkar A (1996) Drying and sintering of sol–gel derived large SiO2 monoliths. J Sol–Gel Sci Technol 6:203–217. https://doi.org/10.1007/BF00402691
Wallace S, Hench LL (1984) The processing and characterization of DCCA modified gel-derived silica. Mater Res Soc Symp Proc 32:47–52. https://doi.org/10.1557/PROC-32-47
Adachi T, Sakka S (1987) Preparation of monolithic silica gel and glass by the sol–gel method using N,N-dimethylformamide. J Mater Sci 22:4407–4410. https://doi.org/10.1007/BF01132038
Hamzaoui HE, Courthéoux L, Nguyen V, Berrier E, Favre A, Bigot L, Bouazaoui M, Capoen B (2010) From porous silica xerogels to bulk optical glasses: the control of densification. Mater Chem Phys 121:83–88. https://doi.org/10.1016/j.matchemphys.2009.12.043
Nakanishi K (1997) Pore structure control of silica gels based on phase separation. J Porous Mater 4:67–112. https://doi.org/10.1023/A:1009627216939
Nakanishi K, Tanaka N (2007) Sol–gel with phase separation. Hierarchically porous materials optimized for high-performance liquid chromatography separations. Acc Chem Res 40:863–873. https://doi.org/10.1021/ar600034p
Siqiang L, Kairong T (1988) Low temperature synthesis of monolithic silica glass from the system Si(OC2H5)4–H2O–HCl–HOCH2CH2OH by the sol–gel method. J Non-Cryst Solids 100:254–262. https://doi.org/10.1016/0022-3093(88)90028-2
Kozuka H, Sakka S (1989) Formation of particulate opaque silica gels from highly acidic solutions of tetramethoxysilane. Chem Mater 1:398–404. https://doi.org/10.1021/cm00004a004
Nakanishi K, Soga N (1991) Phase separation in gelling silica–organic polymer solution: systems containing poly(sodium styrenesulphonate). J Am Ceram Soc 74:2518–2530. https://doi.org/10.1111/j.1151-2916.1991.tb06794.x
Nakanishi K, Komura H, Takahashi R, Soga N (1994) Phase separation in silica sol–gel system containing poly(ethylene oxide). I. Phase relation and gel morphology. Bull Chem Soc Jpn 67:1327–1335. https://doi.org/10.1246/bcsj.67.1327
Kaji H, Nakanishi K, Soga N (1993) Polymerization-induced phase separation in silica sol–gel systems containing formamide. J Sol–Gel Sci Technol 1:35–46. https://doi.org/10.1007/BF00486427
Nakanishi K, Kanamori K (2005) Organic–inorganic hybrid poly(silsesquioxane) monoliths with controlled macro- and mesopores. J Mater Chem 15:3776–3786. https://doi.org/10.1039/b508415f
Tarasevich M (1984) Ultrasonic hydrolysis of a metal alkoxide without alcohol solvents. Am Ceram Soc Bull 63:500
Avnir D, Kaufman VR (1987) Alcohol is an unnecessary additive in the silicon alkoxide sol–gel process. J Non-Cryst Solids 92:180–182. https://doi.org/10.1016/S0022-3093(87)80368-X
Brinker CJ (1998) Hydrolysis and condensation of silicates: effects on structure. J Non-Cryst Solids 100:31–50. https://doi.org/10.1016/0022-3093(88)90005-1
Kajihara K, Hiruta K, Kanamura K (2020) Cosolvent-free sol–gel dip-coating of silica films from tetraalkoxysilane–water binary systems: precursor solutions of long pot life and their characterization by nuclear magnetic resonance spectroscopy. J Ceram Soc Jpn 128:772–782. https://doi.org/10.2109/jcersj2.20150
Kajihara K, Hirano M, Hosono H (2009) Sol–gel synthesis of monolithic silica gels and glasses from phase-separating tetraethoxysilane–water binary system. Chem Commun 2009:2580–2582. https://doi.org/10.1039/b900887j
Kajihara K, Kuwatani S, Maehana R, Kanamura K (2009) Macroscopic phase separation in a tetraethoxysilane-water binary sol–gel system. Bull Chem Soc Jpn 82:1470–1476. https://doi.org/10.1246/bcsj.82.1470
Kuwatani S, Maehana R, Kajihara K, Kanamura K (2010) Amine-buffered phase separating tetraethoxysilane–water binary mixture: A simple precursor of sol–gel derived monolithic silica gels and glasses. Chem Lett 39:712–713. https://doi.org/10.1246/cl.2010.712
Brinker CJ, Keefer KD, Schaefer DW, Ashley CS (1982) Sol–gel transition in simple silicates. J Non-Cryst Solids 48:47–64. https://doi.org/10.1016/0022-3093(82)90245-9
Fidalgo A, Rosa ME, Ilharco LM (2003) Chemical control of highly porous silica xerogels: physical properties and morphology. Chem Mater 15:2186–2192. https://doi.org/10.1021/cm031013p
Kajihara K, Kuwatani S, Kanamura K (2012) Sol–gel synthesis of rare-earth and phosphorus codoped monolithic silica glasses from a cosolvent-free phase-separating system. Appl Phys Express 5:012601. https://doi.org/10.1143/APEX.5.012601
Kaneko K, Kajihara K, Kanamura K (2013) Cosolvent-free sol–gel synthesis of rare-earth and aluminum codoped monolithic silica glasses. J Ceram Soc Jpn 121:299–302. https://doi.org/10.2109/jcersj2.121.299
Suzuki K, Kajihara K, Kanamura K (2014) Cosolvent-free sol–gel synthesis and optical characterization of silica glasses containing LaF3 and (La,Er)F3 nanocrystals. Bull Chem Soc Jpn 87:765–772. https://doi.org/10.1246/bcsj.20140088
Suda M, Nakagawa R, Kanamura K, Kajihara K (2018) Sol–gel-derived transparent silica–(Gd,Pr)PO4 glass-ceramic narrow-band UVB phosphors. Dalton Trans 47:12085–12091. https://doi.org/10.1039/c8dt02998a
Iwasaki R, Kajihara K (2021) Negligible concentration quenching in photoluminescent nanocrystals with high photoactive rare-earth concentrations: silica–(Tb,Ce)PO4 transparent glass-ceramic green phosphors. J Mater Chem C 9:2701–2705. https://doi.org/10.1039/d0tc06043g
Schmidt H, Scholze H, Kaiser A (1984) Principles of hydrolysis and condensation reaction of alkoxysilanes. J Non-Cryst Solids 63:1–11. https://doi.org/10.1016/0022-3093(84)90381-8
Chen KC, Tsuchiya T, Mackenzie JD (1986) Sol–gel processing of silica I. The role of the starting compounds. J Non-Cryst Solids 81:227–237. https://doi.org/10.1016/0022-3093(86)90272-3
Acknowledgements
We wish to thank Mr. Yuki Nishioka and Ms. Rena Asami of Tokyo Metropolitan University for assistance with SEM observations.
Funding
This work was partially supported by JSPS KAKENHI Grant Number 19H02802.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kajihara, K., Goto, M. Cosolvent-free synthesis of macroporous silica gels and monolithic silica glasses from tetraalkoxysilane–water binary systems: comparison between tetramethoxysilane and tetraethoxysilane. J Sol-Gel Sci Technol 104, 497–502 (2022). https://doi.org/10.1007/s10971-022-05799-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05799-9