Abstract
Molecular metal oxo or oxo/alkoxo clusters, MwOx(OH/OR)y(L/X)z (L or X = organic ligands), can often be isolated upon (partial) hydrolysis of metal alkoxides. Investigation of such clusters leads to a better understanding of the basic processes of sol–gel chemistry. The ligands not only stabilize the cluster core but also influence to some extent the cluster structures. They can easily change their position on the cluster surface, thus adapting to changing cluster geometries, and can be exchanged under certain conditions. A close inspection of titanium oxo/alkoxo cluster structures, taken as an informative example, shows that Ti3O units (with or without organic ligands) are the basic building blocks. Clusters with higher nuclearities appear to be predominantly formed by cluster–cluster or by cluster–monomer condensations. Ligand substitution or condensation reactions within a cluster unit are also possible.

Instead of extended metal oxide networks, metal oxo/alkoxo clusters may be formed upon hydrolysis of metal alkoxides.
Highlights
-
Comparison of metal oxo cluster structures allows conclusions on the mechanisms by which the clusters might be formed or modified.
-
Organic ligands protecting the cluster core are dynamic and can be exchanged.
-
Organic ligands influence the cluster structure.
-
Ti3O units are the basic building blocks in titanium oxo clusters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metal alkoxides, M(OR)n, are common precursors in sol–gel chemistry, often modified by neutral (L) or anionic (X) organic ligands. They are converted into the corresponding oxides by means of hydrolysis and condensation reactions. Under certain conditions, however, molecular metal oxo or oxo/alkoxo clusters, MwOx(OH/OR)y(L/X)z, can be isolated instead.
This article is the written version of a talk in the eSeminar series of the International Sol–Gel-Society on November 3, 2021, and will mainly deal with the question what insight can be gained for sol–gel chemistry of metals in general when investigating such oxo/alkoxo clusters. This will be outlined for selected cases (mainly taken from own research), with no intention to cover the existing literature comprehensively.
The clusters are typically synthesized by adding a sub-stoichiometric amount of water to an organically modified or unmodified metal alkoxide (or other hydrolysable metal compounds) and/or by very slow supply of water. There are basically two synthesis protocols: (i) Slow (intended or accidental) water addition to the precursor solution (such as exposure to ambient moisture, hydrated metal salts as precursors, etc.) and (ii) in situ generation of water by a water-producing organic reaction, mostly ester formation, but also aldol condensations and the like.
One of the central questions is whether the clusters are intermediates or side products in the hydrolysis and condensation reactions of metal alkoxides. Connected to that: how are the clusters assembled, how do they grow, and can different cluster types convert into each other? A second group of questions relates to the ligands. “Naked” oxo clusters are not stable, their surface must be protected by ligands. In the clusters discussed in this article, the most obvious type of ligands are residual OR groups. Most clusters also contain other surface groups, mostly anionic ligands, due to their preparation. The denotation “ligands” is justified, because the organic groups capping the cluster cores comply with the principles of coordination chemistry. Two important questions are: are the ligands firmly attached to one particular site at the cluster surface, and to what extent do the ligands influence the cluster structures?
Before coming back to these questions, a brief look at Si/O cage compounds, obtained by hydrolysis of silicon alkoxides or chlorides, SiX4 and R′SiX3 (X = OR or Cl), will mark some important differences between silicon and (transition or main group) metal chemistry.
2 Si/O cage compounds
The equivalent to metal oxo clusters are spherosilicates, [SinOm+x]x− or (R″3SiO)xSinOm, or polyhedral oligomeric silsesquioxanes (POSS, R′xSinOm) (Fig. 1). Already in the 1960s it was proposed that such cage compounds are formed during hydrolysis of R′SiCl3, which then become part of the three-dimensional siloxane networks [1]. Various types of spherosilicates and POSS, with closed or open cages and a variety of functional or non-functional groups R’, have been synthesized since then and are used as valuable building blocks for materials syntheses.
There are two important differences between the silicon-based cage compounds and metal oxo clusters: (i) Silicon is always tetrahedrally coordinated, and interconnected Si tetrahedra only share corners (≡Si–O–Si≡) (Fig. 1, right) rather than edges or faces, and (ii) the groups R′ or OR′ at silicon cannot be exchanged and do not move from one Si to another because they are linked to the core of the Si/O cage through strong covalent bonds. This makes the chemistry of the silicon compounds less diverse than that of metal oxo clusters, although their genesis (partial hydrolysis of SiX4 or R′SiX3) is the same.
3 The ligands
A variety of anionic organic ligands has been used for the modification or functionalization of metal alkoxides or the derived sol–gel materials, such as carboxylates, phosphonates, ß-diketonates, oximates, aminoalcoholates, etc. [2]. Consequently, these groups are also found as capping ligands in metal oxo clusters. Anionic ligands (Xn−) are preferred over neutral ligands (L) because they are more tightly bonded. Neutral ligands, however, may complement the coordination environment of metal atoms on the cluster surface (adduct formation). Anionic organic ligands are mostly bi- or tridentate and can be chelating or bridging. They occupy more than one coordination site, i.e., more than one ligand atom is bonded to the metal(s), and are therefore more strongly bonded than monodentate ligands. In passing, OR groups can also bridge two metals, and thus occupy two coordination sites, as it is well known from many metal alkoxide oligomers. However, since only one atom (the oxygen atom of the OR group) links two metal atoms, the mutual orientation of their coordination polyhedra is different to a situation where the bridging unit consists of more than one atom. We will delve into this subject later on.
A central issue of coordination chemistry is that bonding of coordinatively bonded ligands is, in principle, reversible. This means that the ligands can be decoordinated again, even if the coordination equilibrium may be far on the product side. One of the consequences of this reversibility is that cluster ligands can be exchanged quite easily (as generally in coordination compounds) (Eq. 1).
A second important aspect is that ligands of metal oxo clusters are dynamic [3]. They can change their position on the cluster surface as well as their coordination mode and can therefore easily adjust themselves to a varied cluster core geometry. We will see later that this is important for understanding cluster structures. Finally, the ligands influence the kind of formed cluster due to their electronic and steric properties.
These three issues (reversibility of bonding, ligand dynamics and influence on the cluster structure) will be exemplarily highlighted in the following.
3.1 Ligand dynamics
An often observed phenomenon is that the ligands show only one set of signals in room-temperature n.m.r. spectra, even if they are chemically non-equivalent. This is due to fast intramolecular site exchange processes. An illustrative example is Zr4O2(methacrylate)12 (Fig. 2), obtained from the reaction of Zr(OPr)4 with methacrylic acid. At room temperature only one set of signals was observed for the methacrylate ligands (Fig. 3). The signals broadened by stepwise cooling of the solution. At −10 °C the signals showed a broad coalescence and then split into multiple sets upon further cooling. At −80 °C eight sharp signals of olefinic protons were observed, according to four different sets of ligands (bottom spectrum); the ratio of the four non-equivalent ligands was ~1:1:2:2, indicating the C2h symmetry of the cluster in solution at this temperature, which is in line with the structure in the crystalline state [4].
The cluster Zr4O2(methacrylate)12 with four chemically inequivalent methacrylate ligands (marked with the numbers 1–4) (with permission from ref. [4])
Temperature-dependent 1H NMR spectra of Zr4O2(methacrylate)12 in the CH2 group region (with permission from ref. [4])
Further analysis of the temperature-dependent n.m.r. spectra showed that exchange of the chelating methacrylate (marked 1 in Fig. 2) with the bridging ligands 3 and/or 4 starts at −70 °C and thus has the lowest activation energy. At −60 °C additional exchange with the ligands marked 2 sets in, and at T > −50 °C exchange of all groups.
There are also many cases where such a dynamic behavior of cluster ligands is not observed at room temperature. No obvious relation between the cluster structure, or the coordination of the ligands, and their dynamics is noted. It must be pointed out, however, that n.m.r. investigations at higher temperatures were hardly performed; the onset temperature of the site exchange may just be above ambient temperature.
3.2 Ligand exchange
Post-synthesis ligand exchange is a valuable tool for the modification and functionalization of nanoparticles. This is also possible for metal oxo clusters. However, there are limitations, the most important is that the clusters may rearrange or degrade during ligand exchange (as is also known for nanoparticles).
Capping ligands must compensate the charge of the cluster core and also allow the metal atoms to reach certain coordination numbers. The basic success formula for ligand exchange with retention of the cluster core structure is that the leaving and entering ligand should have the same charge and should occupy the same number of coordination sites. For example, a mono-anionic µ2 ligand should be exchanged for another mono-anionic µ2 ligand. Furthermore, the “bite angle” and related ligand characteristics should be similar. Following this recipe, the methacrylate ligands in Zr4O2(methacrylate)12 were completely replaced by pivalate ligands. Both Zr4O2(OOCR’)12 clusters have the same structure, as proven by X-ray structure analyses. When exposing isolated Zr4O2(pivalate)12 to an excess of methacrylic acid, the original Zr4O2(methacrylate)12 was re-formed [5]. Mixed-ligand clusters can be prepared by partial ligand exchange.
However, even if the aforementioned requirements are met (or pretty much met), cluster rearrangement or degradation may occur. Thus, reaction of the same cluster, viz. Zr4O2(methacrylate)12, with acetylacetone (acac-H) led to complete degradation of the cluster and formation of Zr(acac)2(methacrylate)2 [6]. The lesson learnt from this is that it has to be carefully checked in each case, whether the cluster core structure is retained if one kind of ligand is replaced by another.
3.3 Influence on the cluster structure
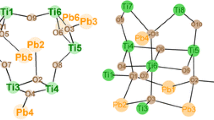
Several studies have shown that cluster formation by partial hydrolysis of metal alkoxides is influenced by basically the same reaction parameters as sol–gel syntheses in general. A prediction which cluster type will be formed in a specific case it is currently not possible owing to the complexity of such reactions. If, however, a particular synthesis protocol is exactly followed, the same cluster can be reliably synthesized in most cases, very often in high yields and in large quantities. Apart from the steric bulk of the OR groups, the M(OR)n/carboxylic acid ratio, the reaction temperature and the solvent (if any), the kind of acid plays an important role. It is not clear until present whether steric or electronic effects (or a combination of both) influence the outcome of the reactions. This influence has been demonstrated in several studies. The example in Fig. 4 is taken from our own work [7] and shows how different cluster types are formed when all reaction parameters are kept constant except the organic group R’ of the acid (in this example the corresponding phosphonic acid is liberated in situ upon reaction of the silyl ester with the employed alcohol). A closer inspection of the cluster structures in Fig. 4 shows, however, that they are constructed from the same building blocks (Ti3O units + single [TiO6] octahedra). This will be discussed in more detail later on.
4 Uses of metal oxo clusters
Apart from the general interest in metal oxo clusters and their relation to sol–gel chemistry—as outlined in the Introduction—some applications are noteworthy. They will only briefly be addressed here without going into details.
1. Clusters are used for crosslinking organic polymers—similar to POSS. To this end, the clusters must be decorated with polymerizable organic groups. Methacrylate ligands are very efficient in this respect, but several other polymerizable or initiator groups have been used as well. A general synthesis protocol is that a mixture of the clusters and organic monomers is co-polymerized. The resulting cluster-reinforced polymers, being organic–inorganic hybrid materials, have interesting materials properties, which were summarized elsewhere [8].
2. Connector units in metal–organic framework structures (MOF) are often metal oxo clusters [9]. The most popular is [Zn4O]6+, but [Zr6O4(OH)4]12+ has also gained some importance [10]. Two approaches are pursued for the synthesis of such MOF. The first is hydrolysis of a molecular precursor, such as ZrOCl2 or Zr(OR)4 in the case of [Zr6O4(OH)4]12+-based MOFs, in the presence of a di-, tri-, or tetracarboxylic acid. The second route is ligand exchange between a pre-formed Zr6O4(OH)4(OOCR′)12 cluster and a polyfunctional carboxylic acid (Fig. 5 as an example).
Synthesis of [Zr6O4(OH)4]12+-based MOFs via reaction of Zr6O4(OH)4(methacrylate)12 with dicarboxylic acids (from ref. [18])
3. Titanium oxo clusters have recently gained some attention because of their optical properties [11].
5 Structures
The structures of more than 350 homometallic titanium oxo clusters (TOC) have been determined. This group of clusters is therefore best suited to discuss exemplarily some structural principles and derive conclusions on the cluster formation [12].
An often repeated claim is that TOC are molecular models for titania. The structures of rutile and anatase may therefore serve as points of reference. Both TiO2 modi-fications have in common that all titanium atoms are octahedrally surrounded by six oxygen atoms ([TiO6] building blocks) and that all oxygen atoms are bonded to three Ti atoms (µ3-O). The basic structural motif is a Ti3O unit, i.e., three [TiO6] octahedra connected via a common µ3-O. However, the shapes of the Ti3O units in rutile and anatase are different (Fig. 6). In rutile, two [TiO6] octahedra share an edge, and the third [TiO6] is connected via a shared corner. This gives a T-shaped Ti3O unit in rutile. In anatase, the three [TiO6] octahedra share two edges, resulting in an L-shaped Ti3O unit. Such Ti3O units are found time and again as basic building blocks in TOCs, as will be highlighted in the following Sections.
The structures of rutile and anatase (reproduced from ref. [19]) and the corresponding Ti3O units
5.1 Oxo–alkoxo clusters
More then ten clusters of the composition TixOy(OR)4x-2y are known, with different sizes and structures, ranging from Ti3O(OR)10 to Ti42O54(OH)18(OiPr)42. Inspection of the structures shows no resemblance of the cluster cores to the TiO2 polymorphs. This is different to other molecular clusters. The cluster cores of many thiolate-capped cadmium sulfide clusters, for example, have the same structure as solid CdS (corner-sharing tetrahedra), and the dangling bonds at the cluster surface are saturated by SR groups [13]. A rationale for the different structural build-up of the Ti/O cluster cores will be given later. However, the TixOy(OR)4x–2y clusters have in common with the TiO2 structures that interconnected Ti3O units are the predominant building blocks (Fig. 7 as an example).
The structure of Ti42O54(OH)18(OiPr)42 [20]. The black circles highlight some of the Ti3O units
5.2 Carboxylate-substituted oxo clusters
Carboxylate-substituted derivatives are the biggest subset among titanium oxo clusters, with ca. 200 structurally characterized examples. This is due to the straightforward synthesis. The basic protocol just requires mixing of Ti(OR)4 and a carboxylic acid. Water is produced in situ through ester formation between the acid and the alcohol, which is liberated by substitution of OR against carboxylate groups.
Titanium atoms in carboxylate-substituted TOCs are mostly 6-coordinate (distorted [TiO6] octahedra), sometimes 5-coordinate, rarely 7- or 4-coordinate. The polyhedra share corners or edges by means of µ2-O, µ2-OR, and µ3-O (rarely OH). Alkoxo groups can be terminal or bridging, carboxylate ligands are always bridging (Fig. 8). Due to the specific binding characteristics of carboxylate ligands (bite angle, Ti–O bond lengths, O–C–O angle, etc.) the [TiOx] polyhedra must be tilted relative to each other. This is an important aspect for understanding TOC structures and also shows how the capping ligands exert an influence on the cluster structure. It also explains that large carboxylato-substituted TOC do not have extended Ti/O cores similar to rutile or anatase: the alignment of the [TiO6] octahedra in the TiO2 modifications is too rigid to allow bridging by carboxylato ligands.
Carboxylato ligands bridging two corner-sharing (left) and edge-sharing [TiO6] octahedra (right). The groups (red dots) connecting the polyhedra can be µ2-O, µ2-OR, and µ3-O (from ref. [12])
Owing to the dual role of carboxylic acids, as a source of the carboxylate ligands and for the production of water in the system, carboxylate-substituted TOCs can be characterized by the R’CO2/Ti ratio (“degree of substitution”, ds) and the O/Ti ratio (“degree of condensation”, dc). A large excess of acid during the synthesis not necessarily results in a high ds, because ester (and water) formation is then also facilitated. A particular combination of ds and dc is therefore currently not predictable. Notwithstanding this, a higher ds results in more open structures as shown for the tetranuclear clusters with the same dc in Fig. 9.
The structures of Ti4O2(OR)10(O2CR’)2 (ds 0.5), and Ti4O2(OR)6(O2CR’)6 (ds 1.5) clusters (adapted from ref. [12])
About 70% of the currently known carboxylate-substituted TOCs are tri-, tetra- or hexanuclear (no pentanuclear derivatives are known). When analyzing the structures of tetra- or hexanuclear clusters, one finds that most can be broken down into Ti3O building blocks, as was the case for the oxo–alkoxo derivatives TixOy(OR)4x–2y. Analysis of the Ti3Ox structures and that of derived compounds thus allows extracting some general principles of cluster formation or modification (It must be emphasized that the following considerations are solely based on the compari-son of cluster structures. It is unknown, whether formation of the clusters during their synthesis goes along the same line). Four “construction principles” can be identified.
5.2.1 Ligand substitution
Although µ2-OR and bridging carboxylate ligands have the same charge and occupy the same number of coordination sites, their structural characteristics are fairly different. Structures which just differ by replacement of a µ2-OR for a carboxylate ligand (with retention of the structure) are therefore infrequent. The example shown in Fig. 10 is exceptional, because a terminal OR group is exchanged for a bridging carboxylate ligand. This is only possible, because one of the titanium atoms in Ti3O(OR)8(O2CR’)2 (left in Fig. 10) is only 5-coordinate which is converted into a 6-coordinate upon substitution. Note that this substitution results in some tilting of the two polyhedra with regard to each other.
The structures of Ti3O(OR)8(O2CR′)2 and Ti3O(OR)7(O2CR′)3 clusters (adapted from ref. [12])
5.2.2 Internal condensation
Two terminal OR groups at neighboring [TiOx] polyhedra can, in principle, undergo an internal condensation reaction. This also requires tilting of the polyhedra relative to each other (while retaining the general cluster core structure) and is therefore also only infrequently found. An example in the Ti3Ox cluster series is Ti3O2(OR)3(O2CR′)5. Its structure can be derived from that of Ti3O(OR)7(O2CR′)3 by both an internal condensation and internal substitutions (Eq. 2).

5.2.3 Cluster–monomer condensation
One can hypothesize that the tetranuclear clusters in Fig. 9 are formed by condensation of a [TiO6] octahedron and a Ti3O unit. In Ti4O2(OR)6(O2CR′)6, the [TiO6] octahedron (on the right-hand side in Fig. 9) is condensed to a T-shaped Ti3O unit via a shared corner and in Ti4O2(OR)10(O2CR′)2 (the lower [TiO6] octahedron in Fig. 9) to an L-shaped Ti3O unit via two shared edges (Remember: R’COO ligands can easily change their position!).
5.2.4 Cluster–cluster condensation
Clusters with the composition Ti6O4(OR)12(O2CR′)4 can have the two structures shown in Fig. 11. The chemical formula and the structures suggest that they might be formed by condensation of two Ti3O(OR)8(O2CR′)2 clusters, either (left in Fig. 11) through condensation via two µ2-O (two shared corners) or (right in Fig. 11) via two µ3-O (one shared edge).
The two structure types of Ti6O4(OR)12(O2CR′)4. The black ellipsoids mark the sites where the two Ti3O subunits are condensed (adapted from ref. [12])
What has been demonstrated for Ti3O clusters as aforesaid, especially (formal) cluster–monomer and cluster–cluster condensations, is found repeatedly for other cluster types. For example, in Ti8O10(O2CR′)12 [14, 15] two Ti4O2(OR)6(O2CR′)6 units are condensed, and in wheel-shaped Ti32O16(OCH2CH2O)32(OR)16(O2CR′)16 [16, 17] eight tetranuclear subunits (=[Ti4O2(OCH2CH2O)4(OR)2(O2CR′)2]8). Cluster–cluster condensations comprise not only the same subunits, but (rarely) also different cluster types. Only few high-nuclearity clusters cannot be broken down into smaller subunits, and even there can Ti3O units be identified.
5.3 Phosphonate-substituted oxo clusters
The main source of the oxide ions in phosphonate-substituted TOC appears to be moisture introduced into the reaction mixture. Although phosphonic acid esters were identified as a side-product in a few reactions of Ti(OR)4 and R″P(O)(OH)2, their formation (and thus water generation in the reaction mixture) is sluggish. Therefore, a mixture of carboxylic and organophosphonic acids is often employed, where the carboxylic acid reacts with the eliminated ROH and produces water, while R″PO3 groups coordinate to Ti. With this protocol, mixed carboxylate-phosphonate-substituted TOCs are often obtained.
Phosphonate ligands have other coordination characteristics than carboxylate ligands. The most important with respect to TOCs are that they (i) can coordinate up to three Ti atoms, (ii) can bridge greater Ti–Ti distances and (iii) have more flexible Ti–O–P angles as well as Ti–O···O–Ti dihedral angles. This results in the coordination possibilities shown in Fig. 12, frequently found in structurally characterized TOC.
Coordination of phosphonate ligands as found in phosphonate-substituted TOC (from ref. [12])
The structural diversity of phosphonate-substituted TOC is smaller than that of carboxylate derivatives. However, there are structural alternatives to the formation of higher-nuclearity TOC merely by condensing Ti/O units. As schematically shown in Fig. 12, R″PO32− ligands can also connect [TiO6] polyhedra without additionally sharing corners or edges. Examples of such structures are given in Fig. 4.
6 Conclusions
Coming back to the questions raised in the Introduction, a potential answer to some (but not all) can be suggested. Taking the formation of Ti/O compounds as an example, there is strong structural (!) evidence that Ti3O clusters are the basic building blocks which are initially formed. The cluster cores may grow by cluster–monomer or cluster–cluster condensation. This has also been postulated for sol–gel materials in general and therefore the cluster may give some hints how the structures of sol–gel materials develop. The clusters can also be modified by substitution or internal condensation reactions. Notwithstanding, several structures of (mainly large) clusters cannot be rationalized by such processes and therefore other growth processes may take place too.
Much more difficult to answer is whether different cluster types can be transformed into each other? Only very few cluster–cluster transformations have been observed experimentally. However, many clusters cannot be re-crystallized. This indicates that equilibria between different species exist in the reaction mixture and isolation of a particular cluster might be controlled by the tendency for crystallization. Experiments for deliberate cluster growth are still lacking, e.g., reaction of an isolated cluster with a cluster of another type or with a metal alkoxide. Although hydrolysis of some metal oxo clusters to give the corresponding metal oxides has been observed, the pathway of such transformations is still unknown. Therefore, the question whether clusters are intermediates in the formation of metal oxides upon hydrolysis of metal alkoxides cannot be answered for sure (although current knowledge suggests that this is the case).
Organic ligands play an important role, not only for controlling the reactivity of metal alkoxides or modifying the properties of sol–gel materials (including metal oxo clusters), but also their structures. There is an interplay between the coordination capabilities of the ligands and the coordination requirements of the metal oxide building blocks. This has been demonstrated for the different structures of carboxylate- and phosphonate-substituted clusters. An analysis of the cluster structures and their n.n.r. spectra also shows that the ligands can change their position at the cluster surface as well as their coordination mode and can therefore easily adapt themselves to changes or extensions of the cluster core.
Deliberate ligand exchange has rarely been investigated in detail. Retention of the cluster core geometry only appears to be feasible without problems, if the bonding characteristics of the incoming and eliminated ligands are similar. Otherwise, one has to reckon with cluster rearrangements.
In summary, investigation of metal oxo clusters obtained by partial hydrolysis of typical sol–gel precursors leads to a better understanding of the basic processes of sol–gel chemistry.
References
Brown Jr. JF (1965) The polycondensation of phenylsilanetriol. J Am Chem Soc 87:4317–4324
Schubert U (2005) Chemical modification of titanium alkoxides for sol-gel processing. J Mater Chem 15:3701–3715
Schubert U (2017) Surface chemistry of carboxylato-substituted metal oxo clusters—model systems for nanoparticles. Coord Chem Rev 350:61–67
Walther P, Puchberger M, Kogler FR, Schwarz K, Schubert U (2009) Ligand dynamics on the surface of zirconium oxo clusters. Phys Chem Chem Phys 11:3640–3647
Kreutzer J, Puchberger M, Artner C, Schubert U (2015) Retention of the cluster core structure during ligand exchange reactions of carboxylato-substituted metal oxo clusters. Eur J. Inorg Chem 2145–2151
Moraru B, Kickelbick G, Batistella M, Schubert U (2001) Degradation of a methacrylate-substituted oxozirconium cluster by acetylacetone. J Organomet Chem 636:172–174
Czakler M, Artner C, Schubert U (2013) Influence of the phosphonate ligand on the structure of phosphonate-substituted titanium oxo clusters. Eur J. Inorg Chem 5790–5796
Schubert U (2011) Cluster-based inorganic-organic hybrid materials. Chem Soc Rev 40:575–582
Tranchemontagne DJ, Mendoza-Cortés JL, O’Keeffe M, Yaghi OM (2009) Secondary building units, nets and bonding in the chemistry of metal–organic frameworks. Chem Soc Rev 38:1257–1283
Bai Y, Dou Y, Xie LH, Rutledge W, Li JR, Zhou HC (2016) Zr-based metal–organic frameworks: design, synthesis, structure, and applications. Chem Soc Rev 45:2327–2367
Zhu QY, Dai J (2021) Titanium oxo/alkoxyl clusters anchored with photoactive ligands. Coord Chem Rev 430:213664
Schubert U (2021) Titanium-oxo clusters with bi- and tridentate organic ligands—gradual evolution of the structures from small to big. Chem Eur J 27:11239–11256
Bendova M, Puchberger M, Pabisch S, Peterlik H, Schubert U (2010) Studies on the formation of CdS nanoparticles from solutions of (NMe4)4[Cd10S4(SPh)16]. Eur J Inorg Chem 2266–2275
Frot T, Marrot J, Sanchez C, Rozes L, Sassoye C (2013) [Ti8O10(OOCR)12] [R = CH(CH3)2 and CCl3] carboxylate titanium oxo-clusters: potential SBUs for the synthesis of metal-organic frameworks. Z Anorg Allg Chem 639:2181–2185
Hong K, Chun H (2013) Nonporous titanium–oxo molecular clusters that reversibly and selectively adsorb carbon dioxide. Inorg Chem 52:9705–9707
Zhao C, Han YZ, Dai S, Chen X, Yan J, Zhang W, Su H, Lin S, Tang Z, Teo BK, Zheng N(2017) Microporous cyclic titanium–oxo clusters with labile surface ligands Angew Chem Int Ed 56:16252–16256
Lv HT, Cui Y, Zou GD, Li N, Yang P, Fan Y (2019) Synthesis of titanium-oxo macrocyles and their catalytic properties for oxidative desulfurization. Dalton Trans 48:14044–14048
Guillerm V, Gross S, Serre S, Devic T, Bauer M, Férey G (2010) A zirconium methacrylate oxocluster as precursor for the low-temperature synthesis of porous zirconium(IV) dicarboxylates. Chem Commun 46:767–769
Coppens P, Chen Y, Trzop E (2014) Crystallography and properties of polyoxotitanate nanoclusters. Chem Rev 114:9645–9661
Gao MY, Wang F, Gu ZG, Zhang DX, Zhang L, Zhang J (2016) Fullerene-like polyoxotitanium cage with high solution stability. J Am Chem Soc 138:2556–2559
Funding
Open access funding provided by TU Wien (TUW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schubert, U. En route from metal alkoxides to metal oxides: metal oxo/alkoxo clusters. J Sol-Gel Sci Technol 105, 587–595 (2023). https://doi.org/10.1007/s10971-022-05774-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05774-4