Abstract
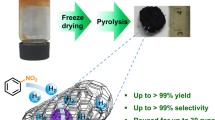
In this work, a new preparation method of highly pure gold aerogel using cellulose triacetate as a template was developed. Gold aerogel with high-purity, low density, and elevated specific surface area was successfully prepared by the proposed fast and simple method as a typical example of noble metal aerogels. In this process, the ligament, crystal grain size, and specific surface area of the product could be controlled by adjusting the concentration of the reducing agent. After complete removal of the template, the obtained gold aerogel with 99% purity showed a moderate catalytic effect toward organic pollutants but with great modification potential. The Raman intensity of pure gold aerogel was 3.2-fold higher than that of the conventional porous gold substrate. The excellent effect of the as-prepared nanogold aerogel was attributed to its enhanced surface plasmon resonance effect induced from high purity. In sum, these findings look promising for future application of high-purity gold aerogels in many fields.







Similar content being viewed by others
References
Bigall NC, Herrmann A-K, Vogel M et al. (2009) Hydrogels and aerogels from noble metal nanoparticles. Angew Chem Int Ed 48(51):9731–9734
Liu W, Herrmann A-K, Bigall NC et al. (2015) Noble metal aerogels-synthesis, characterization, and application as electrocatalysts. Acc Chem Res 48(2):154–162
Quynh BTP, Byun JY, Kim SH (2015) Non-enzymatic amperometric detection of phenol and catechol using nanoporous gold. Sens Actuators B: Chem 221:191–200
Salunkhe RR, Kaneti YV, Yamauchi Y (2017) Metal-organic framework-derived nanoporous metal oxides toward supercapacitor applications: progress and prospects. ACS Nano 11(6):5293–5308
Van ‘T Hag L, Handschin S, Gschwend PM et al. (2020) Light gold: a colloidal approach using latex templates. Adv Funct Mater 30(9):1–9
Asane JK, Qi Z, Biener MM et al. (2018) Optical properties of nanoporous gold foams. AIP Adv 8(9):095302
Xiaocao Z, Haibo Z, Zhibing F et al. (2018) Nanoporous Ni with high surface area for potential hydrogen storage application. Nanomaterials 8(6):394
Sattayasamitsathit S, Thavarungkul P, Thammakhet C et al. (2009) Fabrication of nanoporous copper film for electrochemical detection of glucose. Electroanalysis 21(21):2371–2377
He J, Kunitake T, Watanabe T (2005) Porous and nonporous Ag nanostructures fabricated using cellulose fiber as a template. Chem Commun 6:795–6
Fujita T, Guan P, Mckenna K et al. (2012) Atomic origins of the high catalytic activity of nanoporous gold. Nat Mater 11(9):775–80
Tong H, Ouyang S, Bi Y et al. (2012) Nano-photocatalytic materials: possibilities and challenges. Adv Mater 24(2):229–51
Croix C, Sauvage CE, Balland-Longeau A et al. (2008) New gold-doped foams by copolymerization of organogold(I) monomers for inertial confinement fusion (ICF) targets. J Inorg Organomet Polym Mater 18(3):334–343
Zhang L, Ding Y, Lin Z et al. (2016) Demonstration of enhancement of x-ray flux with foam gold compared to solid gold. Nuclear Fusion 56(3):036006
[1] Li Z, Luo J, Tan X, et al. (2017)X-ray nanotomography characterizations of gold foams. Materials Letters, 205(oct.15):215–218
Dong Y, Yang J, Song T et al. (2018) Efficient soft x-ray sources from laser-irradiated gold foam targets with well-controlled impurities. Nucl Fusion 58(1):016038
Kaur C, Chaurasia S, Borisenko NG, et al. (2019) Demonstration of gold foam plasma as bright x-ray source and slow ion emitters. Plasma Phys Controll Fusion 61(8):4001–4006
Yi Z, Xu X, Li X et al. (2011) Facile preparation of Au/Ag bimetallic hollow nanospheres and its application in surface-enhanced Raman scattering. Appl Surf Sci 258(1):212–217
Xue Y, Scaglione F, Paschalidou EM et al. (2016) Excellent surface enhanced Raman scattering obtained with nanoporous gold fabricated by chemical de-alloying. Chem Phys Lett 665:6–9
Dan W, Wei L, Danny H et al. (2016) Gold aerogels: three-dimensional assembly of nanoparticles and their use as electrocatalytic interfaces. ACS Nano 10(2):2559–2567
Zugic B, Wang L, Heine C et al. (2017) Dynamic restructuring drives catalytic activity on nanoporous gold-silver alloy catalysts. Nat Mater 16(5):558
Zhang K, Tan X, Wu W et al. (2013) Template synthesis of low-density gold foams: density, microstructure and compressive strength. Mater Res Bull 48(9):3499–3504
Liu C, Lian L, Liu Y et al. (2018) Fabrication, characterization of ultra-low-density bulk nanoporous gold with uniform structure and volume shrinkage control during drying Rare Met Mater Eng 47(8):2322–2327
Burpo FJ, Nagelli EA, Morris LA et al. (2017) Direct solution-based reduction synthesis of Au, Pd, and Pt aerogels. J Mater Res 32(22):4153–4165
Burpo FJ, Nagelli EA, Morris LA et al. (2018) A rapid synthesis method for Au, Pd, and Pt aerogels via direct solution-based reduction. J Vis Exp (136):1–13
Fang Y, Chen S, Luo X et al. (2016) Synthesis and characterization of cellulose triacetate aerogels with ultralow densities. RSC Adv 6(59):54054–54059
Cai J, Zhang L (2005) Rapid dissolution of cellulose in LiOH/urea and NaOH/urea aqueous solutions. Macromol Biosci 5(6):539–548
Lee MJ, Lim SH, Ha JM et al. (2016) Green synthesis of high-purity mesoporous gold sponges using self-assembly of gold nanoparticles induced by thiolated poly(ethylene glycol). Langmuir 32(23):5937–45
Krasovskii AN, Vasil’eva IV, Myakin SV et al. (2011) The structure of electron beam-modified cellulose triacetate. High Energy Chem 45(5):390–396
Novikov DV, Krasovskii AN, Mnatsakanov SS (2012) Domain structure of cellulose triacetate. Physics Solid State 54(2):409–413
He J, Kunitake T, Nakao A (2003) Facile in situ synthesis of noble metal nanoparticles in porous cellulose fibers. Chem Mater 15(23):4401–4406
Bigall NC, Herrmann AK, Vogel M et al. (2009) Hydrogels and aerogels from noble metal nanoparticles. Angew Chem Int Ed Engl 48(51):9731–4
Cai J, Wang L, Zhang L (2007) Influence of coagulation temperature on pore size and properties of cellulose membranes prepared from NaOH–urea aqueous solution. Cellulose 14(3):205–215
Li R, Du J, Zheng Y et al. (2017) Ultra-lightweight cellulose foam material: preparation and properties. Cellulose 24(3):1417–1426
Arne W, Andre W, Jürgen B et al. (2011) Nanoporous gold: a new gold catalyst with tunable properties. Faraday Discuss 152:87–98
Wittstock A et al. (2010) Nanoporous gold catalysts for selective gas-phase oxidative coupling of methanol at low temperature. Science 327(15):319–322
Du R, Wang J, Wang Y et al. (2020) Unveiling reductant chemistry in fabricating noble metal aerogels for superior oxygen evolution and ethanol oxidation. Nat Commun 11(1):1590
Deraedt C, Salmon L et al. (2014) Sodium borohydride stabilizes very active gold nanoparticle catalysts. Chem Commun R Soc Chem 50(91):14194–14196
Zhang X, Ding Y (2013) ChemInform abstract: unsupported nanoporous gold for heterogeneous catalysis. ChemInform 44(50):2892–2898
Duan H, Xu C (2015) Low-temperature CO oxidation over unsupported nanoporous gold catalysts with active or inert oxide residues. J Catal 332:31–37
Tang S, Vongehr S, Wang Y et al. (2014) Versatile synthesis of high surface area multi-metallic nanosponges allowing control over nanostructure and alloying for catalysis and SERS detection. J. Mater. Chem. A 2(10):3648–3660
Nahar L, Farghaly AA, Esteves RJA et al. (2017) Shape controlled synthesis of Au/Ag/Pd nanoalloys and their oxidation-induced self-assembly into electrocatalytically active aerogel monoliths. Chem Mater 29(18):7704–7715
Liang W, Y. Yusuke Y (2010) Autoprogrammed synthesis of triple-layered Au@Pd@Pt core-shell nanoparticles consisting of a Au@Pd bimetallic core and nanoporous Pt shell. J Am Chem Soc 132(39):13636–13638
Ibrahem MA, Rasheed BG, Mahdi RI et al. (2020) Plasmonic-enhanced photocatalysis reactions using gold nanostructured films. RSC Adv 10(38):22324–22330
Wang H, Zhang L, Chen Z et al. (2014) Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances. Chem Soc Rev 43(15):5234–44
Takeshi F, Tomoharu T, Ling Z et al. Atomic observation of catalysis-induced nanopore coarsening of nanoporous gold. Nano Lett 14(3):1172–1177
Li J, Cushing SK, Zheng P et al. (2013) Plasmon-induced photonic and energy-transfer enhancement of solar water splitting by a hematite nanorod array. Nat Commun 4:1
Alejandro M, Liu JG, Vikram K et al. (2014) Plasmon-induced hot carriers in metallic nanoparticles. ACS Nano 8(8):7630–7638
Ye W, Yu J, Zhou Y et al. (2016) Green synthesis of Pt–Au dendrimer-like nanoparticles supported on polydopamine-functionalized graphene and their high performance toward 4- nitrophenol reduction. Appl Catal B Environ 181:371–378
Ling Z, Xingyou L, Akihiko H et al. (2011) Wrinkled nanoporous gold films with ultrahigh surface-enhanced Raman scattering enhancement. ACS Nano 5(6):4407–4413
Lingling Y, Guoxiang Z, Hongxin C et al. (2020) Fabrication of gold-silver bimetal nanoparticles/silicon nanoporous pillar array substrate and surface-enhanced Raman scattering detection. Appl Phys A 126(12):957
Yalan L, Li J, Yanqiu Z et al. (2021) Highly reproducible SERS sensor based on self-assembled Au nanocubic monolayer film for sensitive and quantitative detection of glutathione. Appl Surf Sci 540(2):148381
Yun-Xiao Z, Wei-Wei Z, Ye-Yu W et al. (2021) Sensitive surface-enhanced Raman scattering for the quantitative detection of formaldehyde in foods using gold nanorod substrate. Microchem J 160(8):105727
Jeanmaire DL, Duyne RPV (1977) Surface Raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J Electroanal Chemi Interfacial Electrochem 84(1):1–20
Garrell RL, Schultz RH (1985) Surface-enhanced Raman spectroscopy using nonaqueous colloidal silver. J. Colloid Interface Sci 105(2):483–491
Kim JK, Park T-H, Jang D-J (2020) Surface-enhanced Raman scattering and photothermal effect of hollow Au nanourchins with well-defined cavities. J Nanoparticle Res 22(10):305
Xue Y, Scaglione F, Celegato F et al. (2018) Shape controlled gold nanostructures on de-alloyed nanoporous gold with excellent SERS performance. Chem Phys Lett 709:46–51
Acknowledgements
This work was financially supported by the Project of State Key Laboratory of Environment-Friendly Energy Materials of Southwest University of Science and Technology (Grants. 18fksy0203 and 19fksy08), the Open Research Fund Program of Science and Technology on Aerospace Chemical Power Laboratory (Grant. SCTAPL12018B05-1), and the National Natural Science Foundation of China (Grants. 21975066 and 21875061). We thank MogoEdit for its linguistic assistance during the preparation of this manuscript. We also thank Jiayi Zhu for the discussion to this work.
Author contribution
YX: data curation, formal analysis, investigation, methodology, resources, software, writing - original draft. CW: conceptualization, supervision, validation, visualization. KL: writing—review & editing, data curation, methodology, software. ZL and MZ: formal analysis, investigation, project administration. LW: grammar checking, writing—review & editing. YY: funding acquisition, supervision, resources, validation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Xiao, Y., Wang, C., Liu, K. et al. Promising pure gold aerogel: in situ preparation by composite sol–gel and application in catalytic removal of pollutants and SERS. J Sol-Gel Sci Technol 99, 614–626 (2021). https://doi.org/10.1007/s10971-021-05597-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-021-05597-9