Abstract
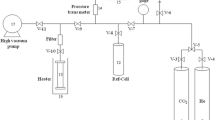
A modification of the MCM-41 mesoporous materials through carbon deposition followed by acid washing has been studied for application in carbon dioxide adsorption. Two routes were used to prepare pure silica (Si-MCM-41) and Al-containing (Al-MCM-41) samples, through hydrothermal synthesis at 150 °C for 24 h. The post-synthesis treatment took place in two stages: carbon deposition using commercial glucose, in the mass ratio of 1 g (support): 2.2 g (glucose): 3.5 g (water), followed by washing with 0.2 M hydrofluoric acid solution at 65 °C for 2 h. The samples were characterized by X-ray diffraction, dispersive energy X-ray spectrometry, nitrogen adsorption–desorption, Fourier-transform infrared spectroscopy, thermal analyzes, and scanning electron microscopy. CO2 adsorption tests were performed on a thermogravimetric balance at 40 °C under atmospheric pressure. Carbon deposition led to a drastic reduction in the surface area and pore volume values of MCM-41 samples, possibly due to deposits of carbonaceous species blocking part of the mesopores and defect sites in the pore wall as self-assembled monolayers. After acid washing, part of these deposits was removed, as well as the occurrence of structural silicon leaching. The post-synthesis treatment allowed the generation of structures with a bimodal pore system, providing a substantial increase in the CO2 adsorption capacity compared to the as-synthesized mesoporous materials (~66% for pure silica and 44% for Al-containing MCM-41 sample), representing a significant improvement in the adsorbents performance for CO2 capture.

Highlights
-
Hydrotermal synthesis of the MCM-41 mesoporous materials (pure silica and Al-containing).
-
Post-synthesis treatment promotes in generation of structures with a bimodal pore system.
-
Post-synthesis treatment results an increase of 44% and 66% in the CO2 adsorption capacity.









Similar content being viewed by others
References
Edmonds J, Reilly JM (1983) Global energy and CO2 to year 2050. Energy J 8(6):419–432
Yang ST, Kim J, Ahn WS (2010) CO2 adsorption over ion-exchange zeolite beta with alkali and alkaline earth metal ions. Micropor Mespor Mater 135(1–3):90–94
Oliver JGJ, Janssens-Maenhout G, Muntean M, Peters JAHW (2013) Trends in global CO2 emissions. PBL Publisher, Hague
Song C (2006) Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal Today 115(1–4):2–32
Figueroa JD, Fout T, Plasynski S, McIlvried H, Srivastava RD (2008) Advances in CO2 capture technology—the U.S. Department of Energy’s Carbon Sequestration Program. Int J Greenh Gas Control 2(1):9–20
Xu X, Song C, Miller BG, Scaroni AW (2005) Adsorption separation of carbon dioxide from flue gas of natural gas-fired boiler by a novel nanoporous molecular basket adsorbent. Fuel Process Technol 86(14–15):1457–1472
Li B, Duan Y, Luebke D, Morreale B (2013) Advances in CO2 capture technology: a patent review. Appl Energy 102:1439–1447
Serna-Guerrero R, Belmabkhout Y, Sayari A (2010) Modeling CO2 adsorption on amine-functionalized mesoporous silica: 1. A semi-empirical equilibrium model. Chem Eng J 161(1–2):173–181
Liu Z, Teng Y, Zhang K, Chen H, Yang Y (2015) CO2 adsorption performance of different amine-based siliceous MCM-41 materials. J Energy Chem 24(3):322–330
Jang HT, Park YK, Ko YS, Lee JY, Margandan B (2009) Highly siliceous MCM-48 from rice husk ash for CO2 adsorption. Int J Greenh Gas Control 3(5):545–549
Yıldıza MG, Davran-Candan T, Günay ME, Yıldırım R (2019) CO2 capture over amine-functionalized MCM-41 and SBA-15: exploratory analysis and decision tree classification of past data. J CO2 Util 31:27–42
Xu X, Song C, Andresen JM, Miller BG, Scaroni AW (2003) Preparation and characterization of novel CO2 “molecular basket” adsorbents based on polymer-modified mesoporous molecular sieve MCM-41. Micropor Mesopor Mater 62(1–2):29–45
Kuwahara Y, Kang DY, Copeland JR, Bollini P, Sievers C, Kaegawa T, Yamashita H, Jones CW (2012) Enhanced CO2 adsorption over polymeric amines supported on heteroatom-incorporated SBA-15 silica: impact of heteroatom type and loading on sorbent structure and adsorption performance. Chem Eur J 18(52):16649–16664
Lin L, Lin W, Zhu YX, Zhao BY, Xie YC, Jia GQ, Li C (2005) Uniformly carbon-covered alumina and its surface characteristics. Langmuir 21(11):5040–5046
Błachnio M, Staszczuk P, Grodzicka G, Lin L, Zhu YX (2007) Adsorption and porosity properties of carbon-covered alumina surfaces. J Therm Anal Calorim 88:601–606
Wang Y, Lin L, Zhu BS, Zhu YX, Xie YC (2008) Different dispersion behavior of glucose and sucrose on alumina and silica surfaces. Appl Surf Sci 254(20):6560–6567
Nascimento RCS, Silva AOS, Meili L (2018) Carbon-covered mesoporous silica and its application in rhodamine B adsorption. Environ Technol 39(9):1123–1132
Silva AE, Ribeiro LMO, Silva BJB, Costa TPM, Meneguetti SMP, Silva AOS (2015) Synthesis and characterization of mesoporous materials containing cerium, lanthanum and praseodymium by nonhydrothermal method. J Sol-Gel Sci Technol 75:413–423
Ozawa T (1965) A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn 38(11):1881–1886
Ozawa T (1966) A new method of quantitative differential thermal analysis. Bull Chem Soc Jpn 39(10):2071–2085
Flynn J, Wall L (1966) General treatment of the thermogravimetry of polymers. J Res Nat Bur Stand 70A(6):487–523
Pedrosa AMG, Souza MJB, Melo DMA, Araujo AS (2006) Cobalt and nickel supported on HY zeolite: synthesis, characterization and catalytic properties. Mater Res Bull 41(6):1105–1111
Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CTW, Olson DH, Sheppard EW, McCullen SB, Higgins JB, Schlenker JL (1992) A new family of mesoporous molecular sieves prepared with liquid crystal templates. J Am Chem Soc 114(27):10834–10843
Kostova NG, Kraleva E, Spojakina AA, Godocikova E, Balaz P (2007) Effect of preparation technique on the properties of Mo-containing Al-MCM-41. J Mater Sci 42:3321–3325
Brahmi L, Ali-Dahmane T, Hamacha R, Hacini S (2016) Catalytic performance of Al-MCM-41 catalyst for the allylation of aromatic aldehydes with allyltrimethylsilane: comparison with TiCl4 as Lewis acid. J Mol Catal A—Chem 423:31–40
Vaschetto EG, Pecchi GA, Casuscelli SG, Eimer GA (2014) Nature of the active sites in Al-MCM-41 nano-structured catalysts for the selective rearrangement of cyclohexanone oxime toward?-caprolactam. Micropor Mesopor Mater 200:110–116
Taib NI, Endud S, Katun MN (2011) Functionalization of mesoporous Si-MCM-41 by grafting with trimethylchlorosilane. Int J Chem 3(3):2–10
Chen H, Wang Y (2002) Preparation of MCM-41 with high thermal stability and complementary textural porosity. Ceram Int 28(5):541–547
Na J, Liu G, Zhou T, Ding G, Hu S, Wang L (2013) Synthesis and catalytic performance of ZSM-5/MCM-41 zeolites with varying mesopore size by surfactant-directed recrystallization. Catal Lett 143:267–275
La-Salvia N, Lovón-Quintana JJ, Lovón ASP, Valença GP (2017) Influence of aluminum addition in the framework of MCM-41 mesoporous molecular sieve synthesized by non-hydrothermal method in an alkali-free system. Mater Res 20(6):1461–1469
Ibrahim M, Alaam M, El-Haes H, Jalbout AF, Leon A (2006) Analysis of the structure and vibrational spectra of glucose and fructose. Eclética Química 31:15–21
Souza MJB, Silva AOS, Aquino JMFB, Fernandes Jr VJ, Araújo AS (2004) Kinetic study of template removal of MCM-41 nanostructured material. J Therm Anal Calorim 75:693–698
Örsi F (1973) Kinetic studies on the thermal decomposition of glucose and fructose. J Therm Anal 5:329–335
Muchan P, Saiwan C, Nithitanakul M (2020) Investigation of adsorption/desorption performance by aminopropyltriethoxysilane grafted onto different mesoporous silica for post-combustion CO2 capture. Clean Energy 4(2):1201–131
Santos TC, Bourrelly S, Llewellyn PL, Carneiro JWM, Ronconi CM (2015) Adsorption of CO2 on amine-functionalised MCM-41: experimental and theoretical studies. Phys Chem Chem Phys 17:11095–11102
Rao N, Wang M, Shang Z, Hou Y, Fan G, Li J (2018) CO2 adsorption by amine-functionalized MCM-41: a comparison between impregnation and grafting modification methods. Energy Fuels 32:670–677
Uyen M, Le T, Lee S-Y, Park S-J (2014) Preparation and characterization of PEI-loaded MCM-41 for CO2 capture. Int J Hydrog Energy 39:12340–12346
Wang X, Guo Q, Zhao J, Chen L (2015) Mixed amine-modified MCM-41 sorbents for CO2 capture. Int J Greenh Gas Control 37:90–98
Kamarudin KSN, Alias N (2013) Adsorption performance of MCM-41 impregnated with amine for CO2 removal. Fuel Process Technol 106:332–337
Liu Z, Teng Y, Zhang K, Cao Y, Pan W (2013) CO2 adsorption properties and thermal stability of different amine-impregnated MCM-41 materials. J Fuel Chem Technol 41(4):469–476
Shen SC, Chen X, Kawi S (2004) CO2 adsorption over Si-MCM-41 materials having basic sites created by postmodification with La2O3. Langmuir 20:9130–9137
Acknowledgements
The authors are grateful for the financial support of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), through a research grant and to CENPES/PETROBRAS and ANP for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, D.P.S., Solano, J.R.S., Sousa, L.V. et al. Modification of MCM-41 type structures by carbon deposition and acid washing for CO2 adsorption. J Sol-Gel Sci Technol 97, 382–392 (2021). https://doi.org/10.1007/s10971-020-05432-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05432-7