Abstract
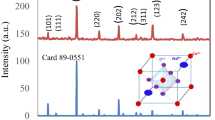
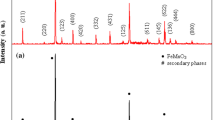
Oxide compound with perovskite-type structure, SrMnO3, was investigated in view of its application as capacitive and/or resistive humidity sensor. The compound presents a porous structure with prevailing open tubular pores systems and it was obtained by sol–gel self-combustion method using polyvinyl alcohol as colloidal medium, followed by heat treatment. Air relative humidity (RH) has a big influence on sensor electric capacity. The best sensitivity as capacitive humidity sensor was found at working frequency of 40 Hz over a wide range of relative humidity (0–98% RH). At this frequency, within the interval 0–98% RH the capacity increases by 1200%, and the resistance decreases by only 18%. The sensor has a good linearity of the logC vs. RH characteristics. The sensor exhibits very small hysteresis, and a short response time. The investigated material holds promise for humidity monitoring applications, taking into account the low cost, a wide range of relative humidity and a low-contamination impact, as well as for the realization of some electronic components, which requires good stability of resistivity in the presence of environmental humidity factors.

Highlights
-
Sol–gel self-combustion can be successfully used for preparation of SrMnO3.
-
In the range 0−98% RH the capacity increases by 1200%, and the resistance decreases by only 18%.
-
The investigated material holds promise for capacitive humidity sensors.
-
Potential candidate for the achievement of electronic components, which requires good stability of resistivity.







Similar content being viewed by others
References
Josephine BA, Manikandan A, Teresita VM, Antony SA (2016) Fundamental study of LaMgxCr1−xO3−δ perovskites nano-photocatalysts: Sol-gel synthesis, characterization and humidity sensing. Korean J Chem Eng 33:1590–1598
Chen Z, Lu C (2005) Humidity sensors: a review of materials and mechanisms. Sens Lett 3:274–295
Vijaya JJ, John Kennedy L, Meenakshisundaram A, Sekaran G, Nagaraja KS (2007) Humidity sensing characteristics of sol-gel derived Sr(II)-added ZnAl2O4 composites. Sens Actuators B Chem 127:619–624
Zou H, Zhang Y, Duan Z, Tong Y, Peng J, Zheng X (2018) Humidity sensing properties of LnFeO3 nanofibers synthesized by electrospinning (Ln = Sm, Nd, La). Mater Res Express 5:015022
Qi T, Levchenko SV, Bennett JW, Grinberg I, Rappe AM (2009) New prospects for high performance SONAR, chemical sensor and communication device materials, HPCMP-UGC 2009: Proceedings of the 2009 DoD high performance computing modernization program users group, IEEE 1:197–204
Shimizu Y, Uemura K, Matsuda H, Miura N, Yamazoe N (1990) Bi‐functional oxygen electrode using large surface area La1−xCaxCoO3 for rechargeable metal‐air battery. J Electrochem Soc 137:3430–3433
Lang X, Mo H, Hu X, Tian H (2017) Supercapacitor performance of perovskite La1−xSrxMnO3. Dalton Transactions 46:13720–13730
Shi W, Ding R, Li X, Xu Q, Ying D, Huang Y, Liu E (2017) Bimetallic Co-Mn perovskite fluorides as highly-stable electrode materials for supercapacitors. Chem Eur J 23:15305–15311
Ding R, Li XD, Shi W, Xu QL, Han XL, Zhou Y, Hong WF, Liu EH (2017) Perovskite KNi0.8Co0.2F3 nanocrystals for supercapacitors. J Mater Chem A 5:17822–17827
Hunter GW, Xu JC, Evans LJ, Vander Wal RL, Gordon M (2006) Chemical sensors based on metal oxide nanostructures, applications. Solid-State Ionic Devices 9:199–209
Doroftei C, Popa PD, Iacomi F (2013) Selectivity between methanol and ethanol gas of La-Pb-Fe-O perovskite synthesized by novel method. Sens Actuators A 190:176–180
Shaterian M, Enhessari M, Rabbani D, Asghari M (2014) Synthesis, characterization and photocatalytic activity of LaMnO3 nanoparticles. Appl Surf Sci 318:213–217
Alifanti M, Kirchnerova J, Delmon B (2003) Effect of substitution by cerium on the activity of LaMnO3 perovskite in methane combustion. Appl Catal A 245:231–244
Tian T, Wang W, Zhan M, Chen C (2010) Catalytic partial oxidation of methane over SrTiO3 with oxygen-permeable membrane reactor. Catal Commun 11:624–628
Rezlescu N, Rezlescu E, Popa PD, Doroftei C, Ignat M (2013) Nanostructured GdAlO3 perovskite, a new possible catalyst for combustion of volatile organic compounds. J Mater Sci 48:4297–4304
Rezlescu N, Rezlescu E, Popa PD, Doroftei C, Ignat M (2015) Some nanograined ferrites and perovskites for catalytic combustion of acetone at low temperature. Ceram Int 41:4430–4437
Aria H, Ezeki S, Shimizu Y, Shippo O, Seiyama T (1983) Semiconductive humidity sensor of perovskite-type oxides. Anal Chem Symp Ser Chem Sens 17:393–398
Leontie L, Doroftei C, Carlescu A (2018) Nanocrystalline iron manganite prepared by sol–gel self-combustion method for sensor applications. Appl Phys A 124:750
Lucaszewicz JP (1991) Diode-type humidity sensor using perovskite-type oxides operable at room temperature. Sens Actuators B 4:227–232
Wang Z, Chen C, Zhang T, Guo H, Zou B, Wang R, Wu F (2007) Humidity sensitive properties of K+ - doped nanocrystalline LaCo0.3Fe0.7O3. Sens Actuators B 126:678–683
Ansari ZA, Ko TG, J.Oh JH (2004) Humidity sensing behavior of thick films of strontium-doped lead-zirconium-titanate. Surf. Coatings Technol 179:182–187
Yeh YC, Tseng TY (1989) Analysis of the d.c. and a.c. properties of K2O-doped porous Ba0.5Sr0.5TiO3 ceramic humidity sensor. J Mater Sci 24:2739–2745
Holc J, Slunecko J, Hrovat M (1995) Temperature characteristics of electrical properties of (Ba,Sr)TiO3 thick film humidity sensors. Sens. Actuators B 26–27:99–102
Doroftei C, Popa PD, Iacomi F (2012) Study of the influence of nickel ions substitutes in barium stannates used as humidity resistive sensors. Sens Actuators A 173:24–29
Ke S, Huang H, Fan H, Chan HLW, Zhou LM (2008) Structural and electric properties of barium strontium titanate based ceramic composite as a humidity sensor. Solid State Ion 179:1632–1635
Wang Y, Park S, Yeow JTW, Langner A, Müller F (2010) A capacitive humidity sensor based on ordered macroporous silicon with thin film surface coating. Sens Actuators B 149:136–142
Traversa E (1995) Ceramic sensors for humidity detection: the State-of-the-art and future developments. Sens Actuators B 23:135–156
Rezlescu N, Rezlescu E, Doroftei C, Popa PD (2005) Study of some Mg-based ferrites as humidity sensors. J Phys: Conf Series 15:296–299
Upadhyay S, Kavitha P (2007) Lanthanum doped stannate for humidity sensor. Mat Letters 61:1912–1915
Madhan K, Murugaraj R (2020) Structural, electrical, and weak ferromagnetic-to-antiferromagnetic nature of Ni and La co-doped BaTiO3 by sol-gel combustion route. J Sol-Gel Sci Technol 95:11–21
Doroftei C, Popa PD, Iacomi F, Leontie L (2014) The influence of Zn2+ ions on the microstructure, electrical and gas sensing properties of La0.8Pb0.2FeO3 perovskite. Sens Actuators B 191:239–245
Doroftei C, Leontie L (2017) Synthesis and characterization of some nanostructured composite oxides for low temperature catalytic combustion of dilute propane. RSC Adv 7:27863–27871
Rezlescu N, Doroftei C, Rezlescu E, Popa PD (2006) Fine grained erbium doped strontium hexaferrite. Phys Stat Sol A 203:3844–3851
Doroftei C, Popa PD, Rezlescu N (2010) The influence of the heat treatment on the humidity sensitivity of magnesium nanoferrite. J Optoelectron Adv Mater 12:881–884
Doroftei C, Leontie L (2019) The influence of Sc3+ ions on the microstructure, electrical, and gas-sensing properties of Ni-Co-Sc ferrite. J Sol-Gel Sci Techn 91:654–663
Doroftei C, Leontie L, Popa A (2017) The study on nanogranular system manganites La-Pb-Ca-Mn-O which exhibits a large magnetoresistance near room temperature. J Mater Sci: Mater Electron 28:12891–12899
Rezlescu N, Popa PD, Rezlescu E, Doroftei C (2008) Microstructure characteristics of some polycrystalline oxide compounds prepared by sol-gel-selfcombustion way for gas sensor applications. Rom J Phys 53:545–555
Leontie L, Doroftei C (2017) Nanostructured spinel ferrites for catalytic combustion of gasoline vapors. Catal Lett 147:2542–2548
Klung H, Alexander L (1962) X-ray diffraction procedures. Wiley, New York
Habibi MH, Mosavi V (2017) Urea combustion synthesis of nano-structure bimetallic perovskite FeMnO3 and mixed monometallic iron manganese oxides: effects of preparation parameters on structural, opto-electronic and photocatalytic activity for photo-degradation of Basic Blue 12. J Mater Sci: Mater Electron 28:8473–8479
Lowell S, Shields JE, Thomas MA, Thommes M (2004) Characterization of porous solids and powders: surface area, pore size and density. Kluwer Academic Publishers, Dordrecht (Boston, London)
Sammes NM, Philipps MB (1993) The synthesis of La1-xSrxMnO3 at different sintering temperatures. J Mater Sci Lett 12:829–830
Zhu D, Zhu H, Zhang Y (2002) Hydrothermal synthesis of La0.5Ba0.5MnO3 nanowires. Appl Phys Lett 80:1634–1636
Khazaei M, Malekzadeh A, Amini F, Mortazavi Y, Khodadadi A (2010) Effect of citric acid concentration as emulsifier on perovskite phase formation of nano-sized SrMnO3 and SrCoO3 samples. Cryst Res Technol 45:1064–1068
Abadian L, Malekzadeh A, Khodadadi A, Mortazavi Y (2008) Effects of excess cobalt oxide nanocrystallites on LaCoO3 catalyst on lowering the light off temperature of CO and hydrocarbons oxidation. Iran J Chem Chem Eng 27:71–77
Søndenå R, Ravindran P, Stølen S (2006) Electronic structure and magnetic properties of cubic and hexagonal SrMnO3. Phys Rev B 74:144102
Ivanov DV, Pinaeva LG, Sadovskaya EM, Isupova LA (2011) Influence of the mobility of oxygen on the reactivity of La1–– xSrxMnO3 perovskites in methane oxidation. Kinetics Catal 52:401–408
Tripathy A, Pramanik S, Cho J, Santhosh J, Osman NAA (2014) Role of morphological structure, doping, and coating of different materials in the sensing characteristics of humidity sensors. Sens 14:16343–16422
Wang J, Wang XH, Wang XD (2005) Study on dielectric properties of humidity sensing nanometer materials. Sens Actuators B 108:445–449
Tripathy A, Pramanik S, Manna A, Bhuyan S, Shah NFA, Radzi Z, N.A.A. Osman NAA (2016) Design and development for capacitive humidity sensor applications of lead-free Ca, Mg, Fe, Ti - Oxides-based electro-ceramics with improved sensing properties via physisorption. Sens 16:1135–1152
Bi H, Yin K, Xie X, Ji J, Wan S, Sun L, Terrones M, Dresselhaus MS (2013) Ultrahigh humidity sensitivity of graphene oxide. Sci Rep 3:2714–2720
Yadav BC, Srivastava R, Dwivedi CD (2008) Synthesis and characterization of ZnO-TiO2 nanocomposite and its application as a humidity sensor. Philos Mag 88:1113–1124
Das J, Hossain SM, Chahraborty S (2001) Role of parasitic in humidity sensing by porous silicon. Sens Actuators A 94:44–52
Wang Z, Shi L, Wu F, Yuan S, Zhao Y, Zhang M (2011) The sol-gel template synthesis of porous TiO2 for a high performance humidity sensor. Nanotech 22:275502–275509
Adamson AW, Gast AP (1997) Physical chemistry of surfaces, 6th ed. Wiley-Blackwell, USA
Gregg SJ, Sing KSW (1982) Adsorption, surface area and porosity, second ed. Academic Press, New York
Agmon N (1995) The Grotthuss mechanism. Chem Phys Lett 244:456–462
Arai H, Ezaki S, Shimizu Y, Shippo O (1983) Semiconductive humidity sensor of perovskite-type oxides. Proc Int Meeting on Chemical Sensors, Fukuoka, Sept. 19–22:393–398
Taguchi H, Takahashi Y, Matsumoto C (1980) The effect of water adsorption on (La1-xSrx)MnO3 (0.1≤x≤0.5). Yogyo Kyokai Shi 88:566–570
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doroftei, C., Leontie, L. Nanocrystalline SrMnO3 perovskite prepared by sol–gel self-combustion method for sensor applications. J Sol-Gel Sci Technol 97, 146–154 (2021). https://doi.org/10.1007/s10971-020-05419-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05419-4