Abstract
Vanadium oxide was synthesized using the non-hydrolytic sol-gel method. Reactions with two different vanadium precursors, VCl3 and VOCl3, were investigated at room temperature. All raw and heat-treated samples were characterized by powder x-ray diffraction, energy-dispersive x-ray spectroscopy coupled with scanning electron microscopy, and thermal analysis. For the VCl3 precursor, crystalline V2O5 was formed following heat treatments between 200 and 250 °C. Broad diffraction features, indicative of nanocrystalline material, were observed in dried samples for VOCl3, while heat treatment to 250 °C produced well-crystallized V2O5. Interesting porous morphologies with large crystallographic coherence lengths were observed for the heat-treated samples.

Highlights
-
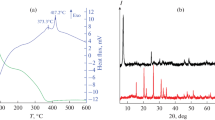
Nanocrystalline V2O5 was synthesized at low temperature using the non-hydrolytic sol–gel method.
-
Well-defined V2O5 crystallized after heating to 200 to 250 °C.
-
Precursor-dependent morphologies include spheres, fibrous webs and platelets.





Similar content being viewed by others
References
Hosokawa S (2016) Synthesis of metal oxides with improved performance using a solvothermal method. J Ceram Soc Jpn 124(9):870–874
Mutin PH, Vioux A (2013) Recent advances in the synthesis of inorganic materials via non-hydrolytic condensation and related low-temperature routes. J Mater Chem A 1(38):11504–11512. https://doi.org/10.1039/C3TA12058A
Danks AE, Hall SR, Schnepp Z (2016) The evolution of “sol-gel” chemistry as a technique for materials synthesis. Mat Horiz 3(2):91–112. https://doi.org/10.1039/c5mh00260e
Corriu RJP, Leclercq D, Lefevre P, Mutin PH, Vioux A (1992) Preparation of monolithic gels from silicon halides by a non-hydrolytic sol-gel process. J Non-Cryst Solids 146(2-3):301–303. https://doi.org/10.1016/s0022-3093(05)80505-8
Acosta S, Corriu RJP, Leclercq D, Lefèvre P, Mutin PH, Vioux A (1994) Preparation of alumina gels by a non-hydrolytic sol-gel processing method. J Non-Cryst Solids 170(3):234–242. https://doi.org/10.1016/0022-3093(94)90052-3
Arnal P, Corriu RJP, Leclercq D, Mutin PH, Vioux A (1997) A solution chemistry study of nonhydrolytic sol-gel routes to titania. Chem Mat 9(3):694–698. https://doi.org/10.1021/cm960337t
Arnal P, Corriu RJP, Leclercq D, Mutin PH, Vioux A (1996) Preparation of anatase, brookite and rutile at low temperature by non-hydrolytic sol-gel methods. J Mat Chem 6(12):1925–1932. https://doi.org/10.1039/jm9960601925
Corriu RJP, Leclercq D, Mutin PH, Vioux A (1995) 29Si nuclear-magnetic resonance study of the structure of silicon oxycarbide glasses derived from organosilicon precursors. J Mat Sci 30(9):2313–2318. https://doi.org/10.1007/bf01184579
Hay JN, Raval HM (2001) Synthesis of organic-inorganic hybrids via the non-hydrolytic sol-gel process. Chem Mat 13(10):3396–3403. https://doi.org/10.1021/cm011024n
Niederberger M (2007) Nonaqueous sol-gel routes to metal oxide nanoparticles. Accounts Chem. Res 40(9):793–800. https://doi.org/10.1021/ar600035e
Niederberger M, Garnweitner G (2006) Organic reaction pathways in the nonaqueous synthesis of metal oxide nanoparticles. Chem-Eur J 12(28):7282–7302. https://doi.org/10.1002/chem.200600313
Vioux A (1997) Nonhydrolytic sol-gel routes to oxides. Chem Mat 9(11):2292–2299. https://doi.org/10.1021/cm970322a
Xu J, Lind C, Wilkinson AP, Pattanaik S (2000) X-ray diffraction and X-ray absorption spectroscopy studies of sol-gel-processed zirconium titanates. Chem Mat 12(11):3347–3355. https://doi.org/10.1021/cm000298s
Andrianainarivelo M, Corriu RJP, Leclercq D, Mutin PH, Vioux A (1997) Non-hydrolytic sol-gel process: Zirconium titanate gels. J Mat Chem 7(2):279–284. https://doi.org/10.1039/a605168e
Debecker DP, Mutin PH (2012) Non-hydrolytic sol-gel routes to heterogeneous catalysts. Chem Soc Rev 41(9):3624–3650. https://doi.org/10.1039/c2cs15330k
Bourget L, Corriu RJP, Leclercq D, Mutin PH, Vioux A (1998) Non-hydrolytic sol–gel routes to silica. J Non-Cryst Solids 242(2):81–91. https://doi.org/10.1016/S0022-3093(98)00789-3
An H, Mike J, Smith KA, Swank L, Lin YH, Pesek SL, Verduzco R, Lutkenhaus JL (2015) Highly flexible self-assembled V2O5 cathodes enabled by conducting diblock copolymers. Sci. Rep. https://doi.org/10.1038/srep14166
Julien C, Haro-Poniatowski E, Camacho-López MA, Escobar-Alarcón L, Jı́menez-Jarquı́n J (1999) Growth of V2O5 thin films by pulsed laser deposition and their applications in lithium microbatteries. Mat Sci Eng B 65(3):170–176. https://doi.org/10.1016/S0921-5107(99)00187-7
Wu NT, Du WZ, Liu GL, Zhou Z, Fu HR, Tang QQ, Liu XM, He YB (2017) Synthesis of hierarchical sisal-like V2O5 with exposed stable {001} facets as long life cathode materials for advanced lithium-ion. Batter ACS Appl Mater Inter 9(50):43681–43687. https://doi.org/10.1021/acsami.7b13944
Cremonesi A, Bersani D, Lottici PP, Djaoued Y, Brüning R (2006) Synthesis and structural characterization of mesoporous V2O5 thin films for electrochromic applications. Thin Solid Films 515(4):1500–1505. https://doi.org/10.1016/j.tsf.2006.04.029
Liu H, Wu YP, Rahm E, Holze R, Wu HQ (2004) Cathode materials for lithium ion batteries prepared by sol-gel methods. J Solid State Electr 8(7):450–466. https://doi.org/10.1007/s10008-004-0521-1
McGraw JM, Perkins JD, Zhang JG, Liu P, Parilla PA, Turner J, Schulz DL, Curtis CJ, Ginley DS (1998) Next generation V2O5 cathode materials for Li rechargeable batteries. Solid State Ion 113:407–413. https://doi.org/10.1016/s0167-2738(98)00383-x
Wu KZ, Sun XL, Duan CY, Gao J, Wu MX (2016) Vanadium oxides (V2O5) prepared with different methods for application as counter electrodes in dye-sensitized solar cells (DSCs). Appl Phys A-Mater Sci Process 122(9):6. https://doi.org/10.1007/s00339-016-0317-z
Sivakumar M, Sakthivel M, Chen SM, Veeramani V, Chen WL, Bharath G, Madhu R, Miyamoto N (2017) A facile low-temperature synthesis of V2O5 flakes for electrochemical detection of hydrogen peroxide sensor. Ionics 23(8):2193–2200. https://doi.org/10.1007/s11581-017-2046-5
Li HY, Yang CH, Tseng CM, Lee SW, Yang CC, Wu TY, Chang JK (2015) Electrochemically grown nanocrystalline V2O5 as high-performance cathode for sodium-ion batteries. J Power Sources 285:418–424. https://doi.org/10.1016/j.jpowsour.2015.03.086
Fang GJ, Liu ZL, Wang Y, Liu YH, Yao KL (2001) Synthesis and structural, electrochromic characterization of pulsed laser deposited vanadium oxide thin films. J Vac Sci Technol a-Vac Surf Films 19(3):887–892. https://doi.org/10.1116/1.1359533
Zhou F, Zhao XM, Yuan CG, Li L, Xu H (2007) Low-temperature hydrothermal synthesis of orthorhombic vanadium pentoxide nanowires. Chem Lett 36(2):310–311. https://doi.org/10.1246/cl.2007.310
Przesniak-Welenc M, Szreder NA, Winiarski A, Lapinski M, Koscielska B, Barczynski RJ, Gazda M, Sadowski W (2015) Electrical conductivity and relaxation processes in V2O5 nanorods prepared by sol-gel method. Phys Status Solidi 252(9):2111–2116. https://doi.org/10.1002/pssb.201552113
Venkatesan A, Chandar NK, Arjunan S, Marimuthu KN, Kumar RM, Jayavel R (2013) Structural, morphological and optical properties of highly monodispersed PEG capped V2O5 nanoparticles synthesized through a non-aqueous route. Mater Lett 91:228–231. https://doi.org/10.1016/j.matlet.2012.09.117
Arnal P, Corriu RJP, Leclercq, D, Mutin PH (1994) Better ceramics through chemistry Vi. In: Cheetham AK, Brinker CJ, Mecartney ML, Sanchez C (eds) Materials research society symposium, Pittsburgh, pp 339–344
Henry A, Hesemann P, Alauzun JG, Boury B (2017) Reductive mineralization of cellulose with vanadium, iron and tungsten chlorides and access to MxOy metal oxides and MxOy/C metal oxide/carbon composites. Carbohyd Polym 174:697–705. https://doi.org/10.1016/j.carbpol.2017.06.106
Diaz C, Barrera G, Segovia M, Valenzuela ML, Osiak M, O’Dwyer C (2015) Crystallizing vanadium pentoxide nanostructures in the solid-state using modified block copolymer and chitosan complexes. J. Nanomater. https://doi.org/10.1155/2015/105157
Chen L, Gu X, Jiang XL, Wang NN, Yue J, Xu HY, Yang J, Qian YT (2014) Hierarchical vanadium pentoxide microflowers with excellent long-term cyclability at high rates for lithium ion batteries. J Power Sources 272:991–996. https://doi.org/10.1016/j.jpowsour.2014.09.048
Modafferi V, Trocino S, Donato A, Panzera G, Neri G (2013) Electrospun V2O5 composite fibers: synthesis, characterization and ammonia sensing properties. Thin Solid Films 548:689–694. https://doi.org/10.1016/j.tsf.2013.03.137
Styskalik A, Skoda D, Barnes CE, Pinkas J (2017) The power of non-hydrolytic sol–gel chemistry: a review. Catalysts 7(6):42. https://doi.org/10.3390/catal7060168
Bretos I, Jiménez R, Ricote J, Calzada ML (2018) Low-temperature crystallization of solution-derived metal oxide thin films assisted by chemical processes. Chem Soc Rev 47(2):291–308. https://doi.org/10.1039/C6CS00917D
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gadient, J., Livingstone, V., Klink, D. et al. Low temperature synthesis of nanocrystalline V2O5 using the non-hydrolytic sol–gel method. J Sol-Gel Sci Technol 89, 663–671 (2019). https://doi.org/10.1007/s10971-019-04926-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-04926-3