Abstract
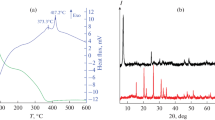
In this study, monoclinic vanadium dioxide (VO2) nanoparticles were synthesized by one-step rapid hydrothermal treatment of a sol precursor, and the obtained products were examined using various analytical methods. Results indicated that monoclinic VO2 nanoparticles could be obtained using ammonium metavanadate as the precursor at a high concentration of 2.37 M and the shortest duration was only 1 h. X-ray diffraction (XRD) revealed that VO2 presented high crystallinity with a pure monoclinic phase. Phase transition latent heat of VO2 was estimated to be 45.5 J/g. Furthermore, VO2 powders were mostly composed of granules with a size distribution ranging from 20 nm to 100 nm. Monoclinic VO2 nanoparticles were derived from the decomposition of the intermediate (NH4)2V4O9.

Monoclinic vanadium dioxide (VO2) nanoparticles can be synthesized by one-step rapid hydrothermal treatment of sol precursor with high concentration.
Highlights
-
The monoclinic vanadium dioxide (VO2) nanoparticles can be synthesized by high-temperature hydrothermal method at above 300 °C.
-
The duration of hydrothermal can be shorten to 1 h.
-
The VO2 nanoparticles could be obtained at a high precursor concentration of 2.37 M.
-
The as-prepared VO2 samples have high phase transition heat and good phase transition performance.










Similar content being viewed by others
References
Wang S, Liu M, Kong L, Long Y, Jiang X, Yu A (2016) Recent progress in VO2 smart coatings: strategies to improve the thermochromic properties. Prog Mater Sci 81:1–54
Gao Y, Luo H, Zhang Z, Kang L, Chen Z, Du J, Kanehira M, Cao C (2012) Nanoceramic VO2 thermochromic smart glass: a review on progress in solution processing. Nano Energy 1:221–246
Granqvist CG, Green S, Niklasson GA, Mlyuka NR, Kræmer SV, Georén P (2010) Advances in chromogenic materials and devices. Thin Solid Films 518:3046–3053
Zou J, Peng Y, Lin H (2013) A low-temperature synthesis of monoclinic VO2 in an atmosphere of air. J Mater Chem A 1:4250–4253
Wu C, Dai J, Zhang X, Yang J, Qi F, Gao C, Xie Y (2010) Direct confined-space combustion forming monoclinic vanadium dioxides. Angew Chem Int Ed 49:134–137
Guiton BS, Gu Q, Prieto AL, Gudiksen MS, Park H (2005) Single-crystalline vanadium dioxide nanowires with rectangular cross sections. J Am Chem Soc 127:498–499
Tian J, Liu F, Shen C, Zhang H, Yang T, Bao L, Wang X, Liu D, Li H, Huang X, Li J, Chen L, Gao H (2007) A new route to single crystalline vanadium dioxide nanoflakes via thermal reduction. J Mater Res 22:1921–1926
Cao C, Gao Y, Luo H (2008) Pure single-crystal rutile vanadium dioxide powders: synthesis, mechanism and phase-transformation property. J Phys Chem C 112:18810–18814
Ji S, Zhao Y, Zhang F, Jin P (2010) Direct formation of single crystal VO2(R) nanorods by one-step hydrothermal treatment. J Cryst Growth 312:282–286
Chen Z, Gao Y, Kang L, Cao C, Chen S, Luo H (2014) Fine crystalline VO2 nanoparticles: synthesis, abnormal phase transition temperatures and excellent optical properties of a derived VO2 nanocomposite foil. J Mater Chem A 2:2718–2727
Dong B, Shen N, Cao C, Chen Z, Luo H, Gao Y (2016) An intermediate phase (NH4)2V4O9 and its effects on the hydrothermal synthesis of VO2 (M) nanoparticles. Cryst Eng Comm 18:558–565
Li W, Ji S, Li Y, Huang A, Luo H, Jin P (2014) Synthesis of VO2 nanoparticles by a hydrothermal-assisted homogeneous precipitation approach for thermochromic applications. RSC Adv 4:13026–13033
Son JH, Wei J, Cobden D, Cao G, Xia Y (2010) Hydrothermal synthesis of monoclinic VO2 micro and nanocrystals in one step and their use in fabricating inverse opals. Chem Mater 22:3043–3050
Li D, Li M, Pan J, Luo Y, Wu H, Zhang Y, Li G (2014) Hydrothermal synthesis of Mo-doped VO2/TiO2 composite nanocrystals with enhanced thermochromic performance. ACS Appl Mater Interfaces 6:6555–6561
Zhu J, Zhou Y, Wang B, Zheng J, Ji S, Yao H, Luo H, Jin P (2015) Vanadium dioxide nanoparticle-based thermochromic smart coating: high luminous transmittance, excellent solar regulation efficiency, and near room temperature phase transition. ACS Appl Mater Interfaces 7:27796–27803
Li W, Ji S, Qian K, Jin P (2015) Preparation and characterization of VO2-BaSO4 composite films with enhanced optical properties in the rmochromic field. Ceram Int 41:5049–5056
Ji S, Zhang F, Jin P (2011) Selective formation of VO2(A) or VO2(R) polymorph by controlling the hydrothermal pressure. J Solid State Chem 184:2285–2292
Zou J, Chen X, Xiao L (2018) Phase transition performance recovery of W-doped VO2 by annealing treatment. Mater Res Express 5:065055
Dai L, Cao C, Gao Y, Luo H (2011) Synthesis and phase transition behavior of undoped VO2 with a strong nano-size effect. Sol Energy Mater Sol Cells 95:712–715
Dong B, Shen N, Cao C, Chen Z, Luo H, Gao Y (2016) Phase and morphology evolution of VO2 nanoparticles using a novel hydrothermal system for thermochromic applications: the growth mechanism and effect of ammonium (NH4+). RSC Adv 6:81559–81568
Manivannan V, Goodenough JB (1998) Low-temperature synthesis of rutile VO2 in aqueous solution using NH2OH.HCl as reducing agent. Mater Res Bull 33:1353–1357
Shen N, Dong B, Cao C, Chen Z, Luo H, Gao Y (2015) Solid-state-reaction synthesis of VO2 nanoparticles with low phase transition temperature, enhanced chemical stability and excellent thermochromic properties. RSC Adv 5:108015–108022
Francavilla J, Chasteen ND (1975) Hydroxide effects on the electron paramagnetic resonance spectrum of aqueous vanadyl(1V) ion. Inorganic Chem 14:2860–2862
Komura A, Hayashi M, Imanaga H (1977) Hydrolytic behavior of oxovanadium(IV) ions. Bull Chem Soc Jap 50:2927–2931
Waal DD, Heyns AM, Range KJ, Eglmeier C (1990) Infrared spectra of the ammonium ion in ammonium metavanadate NH4VO3. Spectrochimica Acta 46A:1639–1648
Acknowledgements
This research was funded by Chongqing Research Program of Basic Research and Frontier Technology (cstc2016jcyjA0027).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zou, J., Xiao, L., Zhu, L. et al. One-step rapid hydrothermal synthesis of monoclinic VO2 nanoparticles with high precursors concentration. J Sol-Gel Sci Technol 91, 302–309 (2019). https://doi.org/10.1007/s10971-019-04999-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-04999-0