Abstract
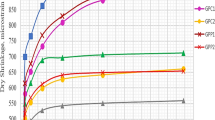
The main target of this work is to investigate the influence of ɣ-Al2O3 on the properties of metakaolin-based geopolymer cements. The kaolin used as starting material for producing geopolymer cements contains approximately 28 and 64% of gibbsite and kaolinite, respectively. This kaolin was transformed to metakaolins by calcination at 500, 550, 600, 650, and 700 °C for 1 h. Gibbsite contained in kaolin was transformed to γ-Al2O3 during the calcination process. The hardener was obtained by mixing commercial sodium silicate and sodium hydroxide solution (10 M) with a mass ratio sodium silicate/sodium hydroxide equal to 1.6:1. Geopolymer cements, GMK-500, GMK-550, GMK-600, GMK-650, and GMK-700, were obtained using the prepared hardener with a mass ratio hardener/metakaolin equal to 0.87:1. It could be seen that the specific surface area of metakaolins decreases with increasing the calcination temperature of kaolin owing to the formation of the particles of γ-Al2O3. The compressive strengths 18.21/29.14/36.61/36.51 increase in the course GMK-550/GMK-600/GMK-650/GMK-700. The X-ray patterns and micrograph images of geopolymer cements, GMK-600, GMK-650, and GMK-700, indicate the presence of γ-Al2O3 in their structure. It was typically found that γ-Al2O3 remains largely unaffected during the geopolymerisation, and therefore could act as an inert filler and reinforce the structure of geopolymer cements.












Similar content being viewed by others
References
Davidovits J (1991) Geopolymers: inorganic polymeric new materials. J Therm Anal Calorim 37:1633–1656
Duxson P, Lukey GC, Separovic F, van Deventer JSJ (2005a) Effect of alkali cations on aluminum incorporation in geopolymeric gels. Ind Eng Chem Res 44:832–839
Rüscher CH, Mielcarek E, Lutz W, Ritzmann A, Kriven WM (2010a) Weakening of alkali-activated metakaolin during ageing investigated by molybdate method and infrared absorption spectroscopy. J Am Ceram Soc 93:2585–2590
Rüscher CH, Mielcarek E, Lutz W, Ritzmann A, Kriven WM (2010b) The ageing process of alkali-activated metakaolin. Ceram Trans 215:1–10
Tchakouté HK, Rüscher CH, Djobo JNY, Kenne BBD, Njopwouo D (2015) Influence of gibbsite and quartz in kaolin on the properties of metakaolin-based geopolymer cements. Appl Clay Sci 107:188–194
Zibouche F, Kerdjoudj H, d’Espinose LJ-B, van Damme H (2009) Geopolymers from Algerian metakaolin. Influence of secondary minerals. Appl Clay Sci 43:453–458
Zhang Z, Wang H, Yao X, Zhu Y (2012) Effects of halloysite in kaolin on the formation and properties of geopolymers. Cem Concr Comp 34:709–715
Rüscher CH, Schulz A, Gougazeh MH, Ritzmann A (2013) Mechanical strength development of geopolymer binder and the effect of quartz content. In: Kriven WM, Wang J, Zhou Y, Gyekenyesi AL, Kirihara S, Widjaja S (Eds.) Developments in strategic materials and computational design IV. Wiley, Hoboken, NJ, http://dx.doi.org/10.1002/9781118807743.ch2
MacKenzie KJD, Temuujin J, Okada K (1999) Thermal decomposition of mechanically activated gibbsite. Thermochim Acta 327:103–108
Favaro L, Boumaza A, Roy P, Ledion J, Sattonnay G, Brubach JB, Huntz AM, Tetot R (2010) Experimental and ab initio infrared study of χ-, k- and α-aluminas formed from gibbsite. J Solid State Chem 183:901–908
Hill MR, Bastow TJ, Celotto S, Hill AJ (2007) Integrated study of the calcination cycle from gibbsite to corundum. Chem Mater 19:2877–2883
Balan E, Lazzeri M, Morin G, Mauri F (2006) First-principles study of the OH-stretching modes of gibbsite. Am Miner 91:115–119
Tchakouté KH, Rüscher CH, Kamseu E, Djobo JNY, Leonelli C (2017) The influence of gibbsite in kaolin and the formation of berlinite on the properties of metakaolin-phosphate-based geopolymer cements. Mater Chem Phys 199:280–288
Brunelle JP, Nortier P, Poisson R (1987) In: Stiles AB (ed) Catalysts and supports catalysts, Butterworth: Boston, 1987, p 11–55
Samain L, Jaworski A, Edén M, Ladd DM, Seo DK, Garcia-Garcia FJ, Häussermann U (2014) Structural analysis of highly porous γ-Al2O3. J Solid State Chem 217:1–8
Barbosa VFF (1999) Sintese e caracterizaçio de polissialatos. PhD Thesis. Instituo Militar de Engenharia, Rio de Janeiro, p 184
Weng L, Sagoe-Crentsil K, Brown T, Song S (2005) Effects of aluminates on the formation of geopolymers. Mater Sci Eng B 117:163–168
Diaz EI, Allouche EN, Eklund S (2010) Factors affecting the suitability of fly ash as source material for geopolymers. Fuel 89:992–996
Swanepoel JC, Strydom CA (2002) Utilization of fly ash in a geopolymeric material. Appl Geochem 17:1143–1148
Lee WKW, van Deventer JSJ (2002) The effects of inorganic salt contamination on the strength and durability of geopolymers. Colloids Surf A 212:27–44
Criado M, Palomo A, Fernandez-Jimenez A (2005) Alkali activation of fly ash. Part 1: effect of curing conditions on the carbonation of the reaction products. Fuel 84:2048–2054
Rocha J, Klinowski J (1990) 295i and 27Al Magic-angle-spinning NMR studies of the transformation of kaolinite. Phys Chem Miner 17:179–186
Maia AÁB, Angélica RS, de Freitas Neves R, Pöllmann H, Straub C, Saalwächter K (2014) Use of 29Si and 27Al MAS NMR to study thermal a ctivation of kaolinites from B razilian Amazon kaolin wastes Appl Clay Sci 87:189–196
Klinowski J (1884) Nuclear magnetic resonance studies of zeolites. Prog NMR Spectrosc 16:237–309
Engelhardt G, Michel D (1987) High-resolution solid-state NMR of silicates and zeolites. Wiley, Chichester West Sussex and New York
Ahn SW, Lee HS, Yang WH, Jeong YK (2011) 27Al MAS-RMN study of inorganic polymer formation at ambient temperature. Trans Nonferrous Met Soc China 21:182–187
Acknowledgements
Hervé Tchakouté Kouamo gratefully acknowledges the Alexander von Humboldt Foundation for financial support the purchase of equipment under the grant N° KAM/1155741 STP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Highlights
-
Kaolin containing gibbsite and kaolinite is applied for producing geopolymer cements.
-
Gibbsite contained in kaolin was transformed to γ-Al2O3 during the calcination process.
-
The specific surface area of metakaolins decreases with increasing the calcination temperature.
-
γ-Al2O3 remains largely unaffected during the geopolymerization.
-
γ-Al2O3 could act as filler and reinforces the structure of geopolymer cements.
Rights and permissions
About this article
Cite this article
Tchakouté, H.K., Kamseu, E., Banenzoué, C. et al. Role of ɣ-Al2O3 on the mechanical and microstructural properties of metakaolin-based geopolymer cements. J Sol-Gel Sci Technol 86, 305–315 (2018). https://doi.org/10.1007/s10971-018-4616-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-018-4616-z