Abstract
Adsorption characteristics of Fe(III) ions onto thiocyanato-functionalized silica gel sorbent were investigated under various conditions of contact time, initial metal concentration and temperature. The equilibrium isotherms, kinetics and thermodynamics of Fe(III) ions adsorption onto the surface of thiocyanato-functionalized silica gel from aqueous solution were investigated. Batch kinetic studies showed that an equilibrium time of 30 min was required for the adsorption of Fe(III) ions onto thiocyanato-functionalized silica gel. The estimated maximum capacities of Fe(III) ions adsorbed by thiocyanato-functionalized silica gel were 112.2 mg g−1. The equilibrium adsorption data of thiocyanato-functionalized silica gel for Fe(III) ions best fitted to the Langmuir isotherm since the correlation coefficients for the Langmuir isotherm were higher than that for the Freundlich and Dubinin–Radushkevich isotherms. The kinetic data were well described by the pseudo-second-order model. Spontaneous, endothermic and random characteristics of the process were confirmed by thermodynamic analysis.
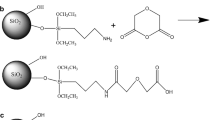
Graphical Abstract








Similar content being viewed by others
References
Noubactep C (2010) Metallic iron for safe drinking water worldwide. Chem Eng J 165:740–749
Ghosh D, Solanki H, Purkait MK (2008) Removal of Fe(II) from tap water by electrocoagulation technique. J Hazard Mater 155:135–143
Yavuz H, Say R, Denizli A (2005) Iron removal from human plasma based on molecular recognition using imprinted beads. Mater Sci Eng C 25:521–528
Litovitz TL, Schmitz BF, Matyunas N, Martin TG (1988) Annual report of the American association of poison control centers national data collection system. Am J Emerg Med 6:479–515
Azher NE, Gourich B, Vial C, Soulami MB, Ziyad M (2008) Study of ferrous iron oxidation in Morocco drinking water in an airlift reactor. Chem Eng Process 47:1877–1886
Owens GS, Southard GE, Van Houten KA, Murray GM (2005) Molecularly imprinted ion-exchange resin for Fe3+. Sep Sci Technol 40:2205–2211
Berbenni P, Pollice A, Canziani R, Stabile L, Nobili F (2000) Removal of iron and manganese from hydrocarbon-contaminated groundwaters. Bioresour Technol 74:109–114
Andersen WC, Bruno TJ (2003) Application of gas–liquid entraining rotor to supercritical fluid extraction: removal of iron(III) from water. Anal Chim Acta 485:1–8
Choo K-H, Lee H, Choi S-J (2005) Iron and manganese removal and membrane fouling during UF in conjunction with prechlorination for drinking water treatment. J Membr Sci 267:18–26
Ellis D, Bouchard C, Lantagne G (2000) Removal of iron and manganese from groundwater by oxidation and microfiltration. Desalination 130:255–264
Reiner D, Poe DP, Chem A (1977) Removal of iron, copper, cadmium, cobalt, and nickel from sodium hydroxide by precipitation and extraction with phenyl-2-pyridyl ketoxime. Anal Chem 49:889–891
Karabörk M, Ersöz A, Denizli A, Say R (2008) Polymer-clay nanocomposite iron traps based on intersurface ion-imprinting. Ind Eng Chem Res 47:2258–2264
Okoniewska E, Lach J, Kacprzak M, Neczaj E (2007) The removal of manganese, iron and ammonium nitrogen on impregnated activated carbon. Desalination 206:251–258
Correa-Murrieta MA, López-Cervantes J, Sánchez-Machado DI, Sánchez-Duarte RG (2014) Synthesis and application of modified chitosan beads for iron removal: kinetic and isotherm models. Asia Pac J Chem Eng 9:895–904
Senin HB, Subhi O, Rosliza R, Kancono N, Azhar MS, Hasiah S, Wan Nik WB (2006) Role of sawdust in the removal of iron from aqueous solution. Asean J Sci Technol Dev 23:223–229
Beenakumari KS (2009) Removal of iron from water using modified coconut shell charcoal as adsorbent. Curr World Environ 4:321–326
Gedamy YR (2013) Successive consecutive removal of some heavy metals from groundwater of the quaternary aquifer in El-Minya governorate using rice straw. J Appl Sci Res 9:4299–4317
Bun-Ei R, Kawasaki N, Ogata F, Nakamura T, Aochi K, Tanada S (2006) Removal of lead and iron ions by vegetable biomass in drinking water. J Oleo Sci 55:423–427
Yeddou N, Bensmaili A (2007) Equilibrium and kinetic modelling of iron adsorption by eggshells in a batch system: effect of temperature. Desalination 206:127–134
Prakash S, Mohanty JK, Das B, Venugopal R (2001) Characterisation and removal of iron from fly ash of Talcher area, Orissa, India. Miner Eng 14:123–126
Golden TC, Hsiung TH, Snyder KE (1991) Removal of trace iron and nickel carbonyls by adsorption. Ind Eng Chem Res 30:502–507
Qian G, Li M, Wang F, Liu X (2014) Removal of Fe3+ from aqueous solution by natural apatite. J Surf Eng Mater Adv Technol 4:14–20
Wang Y, Sikora S, Kim H, Bonzongo J-C, Rhue D, Townsend TG (2013) Evaluation of mineral substrates for in situ iron removal from groundwater. Environ Earth Sci 69:2247–2255
Cui A, Singh A, Kaplan DL (2002) Enzyme-based molecular imprinting with metals. Biomacromolecules 3:1353–1358
Özkara S, Say R, Andac C, Denizli A (2008) An ion-imprinted monolith for in vitro removal of iron out of human plasma with beta thalassemia. Ind Eng Chem Res 47:7849–7856
Dey RK, Jha U, Patnaik T, Singh AC, Singh VK (2009) Removal of toxic/heavy metal ions using ion-imprinted aminofunctionalized silica gel. Sep Sci Technol 44:1829–1850
Fan H-T, Sun T (2012) Selective removal of iron from aqueous solution using ion imprinted cyanato-functionalized silica gel sorbents. Sep Sci Technol 47:507–512
Fan H-T, Su Z-J, Fan X-L, Guo M-M, Wang J, Gao S, Sun T (2012) Sol–gel derived organic–inorganic hybrid sorbent for removal of Pb2+, Cd2+ and Cu2+ from aqueous solution. J Sol–Gel Sci Technol 64:418–426
Fan H-T, Tang Q, Sun Y, Zhang Z-G, Li W-X (2014) Selective removal of antimony(III) from aqueous solution using antimony(III)-imprinted organic–inorganic hybrid sorbents by combination of surface imprinting technique with sol–gel process. Chem Eng J 258:146–156
Chie M (1960) Magnetic and spectrophotometric studies on decoloration of thiocyanato iron(III) complexes in solutions. I Bull Chem Soc Jpn 33:867–871
Raghunandan S, Prasad CL, Kumar MR (1986) Determination of iron in drug biochemical and biological samples as a thiocyanato mixed ligand complex with hydroxyamidine. Bull Chem Soc Jpn 59:1571–1573
Fan H-T, Sun T (2012) Selective removal of iron from aqueous solution using ion imprinted thiocyanato-functionalized silica gel sorbents. Korean J Chem Eng 29:798–803
Blitz IP, Blitz JP, Guńko VM, Sheeran DJ (2007) Functionalized silicas: structural characteristics and adsorption of Cu(II) and Pb(II). Colloids Surf A 307:83–92
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Freundlich HMF (1906) Über die adsorption in lösungen. Z Phys Chem 57:385–470
Dubinin MM, Radushkevich LV (1947) Equation of the characteristic curve of activated charcoal. Chem Zentr 1:875
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetensk Handl 24:1–39
Ho YS, McKay G (1999) Pseudo-second-order model for sorption processes. Process Biochem 34:451–465
Low MJD (1960) Kinetics of chemisorption of gases on solids. Chem Rev 60:267–312
Weber WJ, Morris JC (1963) Kinetics of adsorption of carbon from solutions. J Sanit Eng Div Am Soc Civ Eng 89:31–63
Chamberlain MM, Bailar JC (1959) The infrared spectra of some thiocyanatocobalt ammines. J Am Chem Soc 81:6412–6415
Al-Oweini R, El-Rassy H (2009) Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si(OR)4 and R”Si(OR’)3 precursors. J Mol Struct 919:140–145
Fan H-T, Wu J-B, Fan X-L, Zhang D-S, Su Z-J, Yan F, Sun T (2012) Removal of cadmium(II) and lead(II) from aqueous solution using sulfur-functionalized silica prepared by hydrothermal-assisted grafting method. Chem Eng J 198–199:355–363
Özcan A, Özcan AS (2005) Adsorption of acid red 57 from aqueous solutions onto surfactant-modified sepiolite. J Hazard Mater 125:252–259
Kannan K, Sundaram MM (2001) Kinetics and mechanism of removal of methylene blue by adsorption on various carbon—a comparative study. Dyes Pigm 51:25–40
Tajar AF, Soleimani TKM (2009) Adsorption of cadmium from aqueous solutions on sulfurized activated carbon prepared from nut shells. J Hazard Mater 165:1159–1164
Zheng H, Wang Y, Zheng Y, Zhang H, Liang S, Long M (2008) Equilibrium, kinetic and thermodynamic studies on the sorption of 4-hydroxyphenol on Cr-bentonite. Chem Eng J 143:117–123
Javadian H, Ghaemy M, Taghavi M (2014) Adsorption kinetics, isotherm, and thermodynamics of Hg2+ to polyaniline/hexagonal mesoporous silica nanocomposite in water/wastewater. J Mater Sci 49:232–242
Fan H-T, Sun Y, Tang Q, Li W-L, Sun T (2014) Selective adsorption of antimony(III) from aqueous solution by ion-imprinted organic–inorganic hybrid sorbent: kinetics, isotherms and thermodynamics. J Taiwan Inst Chem Eng 45:2640–2648
United States Environmental Protection Agency (2000) Drinking Water Standards and Health Advisories, United States Environmental Protection Agency. http://www.epa.gov/OST. EPA 822-B-00-001, Office of Water, Washington, DC
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sui, DP., Chen, HX. & Li, DW. Sol–gel-derived thiocyanato-functionalized silica gel sorbents for adsorption of Fe(III) ions from aqueous solution: kinetics, isotherms and thermodynamics. J Sol-Gel Sci Technol 80, 504–513 (2016). https://doi.org/10.1007/s10971-016-4092-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-016-4092-2