Abstract
Undoped and Pb2+-doped ultrafine cubic zinc aluminate (ZnAl2O4) hosts were successfully prepared at a relatively low temperature (~80 °C) using the sol–gel method. The concentration of Pb2+ was varied from 0 to 5 mol%. The TGA showed that the minimum annealing temperature required to obtain single phase ZnAl2O4 must be above 400 °C. The XRD data revealed that all the annealed samples were single phase crystalline structures and the estimated crystallites size were in the range of 21–30 nm in diameter. The FTIR results suggest that heat-treating can destroy some of the bonds. The surface morphology of the phosphors was influenced by the Pb2+ mol%. Undoped and Pb2+-doped ZnAl2O4 nanoparticles exhibit the violet emission at slightly different positions. The slight peak shifts suggests the possibilities that the luminescence centre can either be due to the defects level in the host or Pb2+ ions. The emission peaks at 390 and 399 nm are ascribed to the typical UV transitions 3P0,1 → 1S0 in Pb2+ ion. At the higher Pb2+ mol%, the luminescence quenching behaviour occurs, which suggests that doping with Pb2+ ions is accompanied by the introduction of new defect sites that enhance non-radiative recombination of the excited electrons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Doped and undoped semiconductor nanoparticles have received major interest in the scientific community because of their unique and superior properties, such as size-dependent optical properties [1]. ZnAl2O4 is one of the wide-band gap (~3.8 eV) semiconductors being considered for the photoelectronic devices, UV-transparent conductor, sensor, and dielectric material [2–4]. ZnAl2O4 have the chemical formula AB2O4, where A is Zn2+ ion that occupies a tetrahedral and B represent Al3+ ion which occupies octahedral sites of a cubic crystal [5]. ZnAl2O4 and other spinels are known to be suitable host lattice for various dopants or activator atoms [6, 7]. For instance, there have been several reports on the rare earth and transition metal ions doped ZnAl2O4 phosphor, and most of these studies focus more on the well known luminescing dopants such as Mn2+, Eu2+, Tb3+, etc. [3]. Apart from the rare earths and transition metal, a group of so called heavy ns 2 metal ions such as Tl+, Pb2+ and Bi3+ luminescing dopants have been studied in a considerable number of hosts [8, 9]. In particular, the luminescence of the Pb2+ ion is quite diverse and strongly depends on the environmental condition of Pb2+ in a host lattice [10, 11]. The nature of Pb2+ ion luminescence in complex oxides usually consists of emission bands in the UV and visible ranges [12]. The UV emission is ascribed to the 3P0,1,2 → 1S0 transition in the Pb2+ monomer center. While the visible emission is ascribed to emission from the D-level, which is believed to originate from a charge transfer transition [10, 13].Many fabrication methods of ZnAl2O4 phosphor such as solid state reaction [14], co-precipitation [15], hydrothermal [16], combustion [17] and sol–gel [18] have been developed. As an alternative, in this work, we have adopted the sol–gel method [18], which is known to be a low-temperature technique, in order to obtain a low cost green phosphor for use in applications such as X-ray imaging device, low pressure lamps, and high-energy physics [18]. In view of these, this paper, reports on the sol–gel synthesis of undoped and Pb2+-doped ZnAl2O4 at a very low temperature (~80 °C). The effects of Pb2+ content at a range of 0–5 mol% on the structural and luminescence properties were investigated with the aim of fabricating UV down-conversion phosphor materials that can be used in different types of light emitting devices.
2 Experimental
The undoped and Pb2+-doped ZnAl2O4 nanocrystals were synthesized using a well-known sol–gel technique [18]. Zn(NO3)2·6H2O (98 %), Al(NO3)3·9H2O (98.5 %) and Citric acid, C8H8O7·H2O (99 %) were dissolved in deionized water. The sols stoichiometric molar ratio of Zn:Al was 2:1. Specified amounts of Pb(NO3)2 was added in the solution to dope with different mol% of Pb2+ ions. The Pb2+ content was varied at the range of 0–5 mol%. In all samples, the heating temperature was kept below 80 °C while constantly stirring on a magnetic stirrer until the transparent gels of the mixed metal citrates were formed. The gels were dried at room temperature for 2 days and then in an oven kept at a temperature of 145 °C. The white dried gels were ground into fine powders and were subsequently annealed at 800 °C in a furnace for an hour. The samples were then taken for the characterization. Thermogravimetric analysis was carried out using a Perkin Elmer TGA7 in the temperature range of 20–580 °C at a heating rate of 10 °C/min under an air flux. The stretching mode frequencies were determined using a Perkin Elmer Spectrum 100 FTIR spectrometer. The crystal structure of the samples was characterized by X-ray powder diffraction (Bruker AXS Discover diffractometer) with CuKα (1.5418 Å) radiation). The surface morphology of the phosphor powders was analyzed using a Shimadzu Superscan ZU SSX-550 scanning electron microscope (SEM). Photoluminescent spectra were recorded at room temperature using a Hitachi F-7000 fluorescence spectrophotometer.
3 Results and discussion
3.1 Thermogravimetric analysis
Figure 1 shows the thermal decomposition of the as prepared ZnAl2O4 (host) gel. Several thermal features take place during the heating of the gel. As anticipated the variation of the mass of the compound with temperature indicates slow weight loss up to 80 °C due to the release of interlayer water [4, 19]. An event at around 150–200 °C, is attributed to the expulsion of hydrated water [4, 19, 20]. The last event at around 300–400 °C is attributed to the elimination of organic compounds and starting of crystallization process and the formation of single phase ZnAl2O4 [4, 19].
These results suggest that the minimum annealing temperature to start obtaining cubic phase ZnAl2O4signature is 400 °C with high chances of forming other oxides impurities such as ZnO and Al2O3. Thus, the annealing temperature must be higher than 400 °C to completely eradicate impurities and enhances the crystallinity of the single phase ZnAl2O4. TGA results are consistent with what is observed in the XRD and FTIR analysis.
3.2 X-ray diffraction
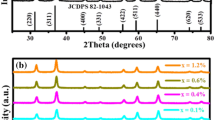
The crystallinity of the powder samples prepared by sol–gel method was determined by means of XRD. To reveal the structural properties of the un-doped and Pb2+doped ZnAl2O4, the XRD patterns are presented in Fig. 2. The XRD pattern is consistent with the standard data for the cubic ZnAl2O4 spinel phase (JCDPS: 82-1043). The results clearly reveal that highly crystalline cubic ZnAl2O4 single phase can be obtained after annealing at 800 °C. This is supported by the fact that there were no extra peaks of impurities related to ZnO or Al2O3. The same phases without impurities were observed for all Pb2+doped samples at the range of 0–5 mol%. Thus, it is highly believed that the Pb2+ ions were fully incorporated into the host matrix. The results suggest that varying the mol% of Pb2+ ions does not affect the crystal structure of the phosphor. The crystallites sizes were estimated by using the Scherrer formula [21], using the most intense peak, and were found to be 22, 29, 21, 30 and 22 nm for the 0, 0.25, 1, 3 and 5 % Pb2+ ions, respectively. Furthermore, when considering the most intense diffraction peak (311) shown in Fig. 2b, it can be seen that there is a peak shift to the higher angle and decrease in diffraction intensity with an increase in Pb2+ mol% concentration. This relationship in our results is consistent with what has been reported by Hou et al. [22] in the study of electrical and optical properties of Al doped ZnO and ZnAl2O4. The average lattice spacing was estimated to be 0.24 nm, which corresponds with (311) lattice spacing of the ZnAl2O4 reported by Zawadzki et al. [23]. As expected, shifting to the higher angle is attributed to the decrease in the lattice spacing as the Pb2+ ions are incorporated into the ZnAl2O4 matrix (see Fig. 2c). This is due to the substitution of Zn2+ (ionic radius 0.74 Å) [22] with bigger Pb2+ (ionic radius 1.01–1.40 Å) [24] ions. In general, it is assumed that when a cation is replaced by other larger, the result is an expansion of the lattice and therefore both the interplanar d-spacing increases and the 2θ is shifted to lower angle. So, the mechanism of Pb2+-doped ZnAl2O4 solid solution formation must be different. May be it could include anionic vacancy formation.
Lubarda et al. [25] observed similar results deviating from Vergard’s law when replacing Au atoms (1.5939 Å) with Ag atoms (1.5969 Å) in the Ag–Au alloy. The variation of the lattice spacing of the (311) diffraction peak, shows (in Fig. 2c) the parabolic behaviour relationship to the Pb2+ mol%. The decrease in lattice spacing in our study is, therefore, attributed to the shrinkage of the Pb2+ outer electron shell due to its electronic interactions with more neighbouring Zn2+ ions, which makes it smaller than the Zn2+ ion [25]. As the Pb2+ mol% is increased further, the shrinkage is expected to be less pronounced (due to the additional Pb2+ ions), as a results, the lattice spacing is expected to recover or increase and this serves as a motivation why there is an increase in lattice spacing at 5 % Pb2+. These can also be a relevant reason why Lubarda et al. [25] observed high increase in lattice spacing at higher mol% of the Ag. Lubarda et al. [25] results and our results suggest that for some materials, at a low mol% of the dopant, the Vergard’s law is not favoured. Thus, it is concluded that there is an optimum mol% required for the Vergard’s law to occur. The second order polynomial fit in Fig. 2c shows that the 3.4 % Pb2+ is an optimum for our results to behave as expected by Vergard’s law. The decrease of the (311) diffraction peak intensity as the Pb2+ mol% is increased can be explained to be due to the replacement of smaller with bigger atoms, which destroys the crystal quality. The destruction of crystal quality is expected to be more pronounced as bigger foreign atoms are incorporated and this is supported by our findings in Fig. 2b. It is therefore concluded that the Pb2+ mol% influences the particle size growth, the lattice spacing and crystal quality.
3.3 FTIR
Figure 3 shows the FTIR spectra of the un-doped ZnAl2O4 gel and annealed at 800 °C powder. The band at 682 cm−1 is assign to the stretching vibration of tetrahedral and octahedral bonds in the spinel [4, 26, 27]. The IR spectra of the gel indicate the presence of nitrates groups at 805, 897 and 1,052 cm−1. The bands at 1,330–1,628 cm−1 can be attributed to the OH group in the metal alkoxides present in the gel [27, 28]. The wide absorption band centred at 2,965–3,520 cm−1 corresponds to OH group, which is contributed by the water content present in the gel. As far as the annealed sample is concern, the shallow band observed at 3,581 cm−1 is assigned to O–H stretching vibration [29]. The band at 2,337 cm−1 is attributed to the presence of O–O bonds in the crystal lattice, which is treated as a feature of spinel structured crystallites. Finally, a small band at 1,545 cm−1 is attributed to the amide bonds [30]. In comparing the gel and the annealed powder, it can be seen that the number of bands decreases when the samples were annealed at 800 °C, suggesting that the annealing temperature destroys most of the bonds, which suggests the formation of the single phase ZnAl2O4 as shown by the XRD results.
3.4 SEM
The surface morphologies of the samples annealed at 800 °C for the undoped, 1 and 5 % Pb2+-doped ZnAl2O4 are shown on SEM micrographs in Fig. 4a–c, respectively. The undoped powder composed of small spherical and chunk granular particles dispersed all over the surface. The 1 % Pb2+-doped sample shows rough particle agglomerates with voids distributed in the matrix. It is, therefore, proposed that the rough particle agglomerates with voids might lead to easy escape routes to the emitted light with the consequent increase in the light output. The 5 % Pb2+doped sample shows the particle-to-particle separation and more grain boundaries-like-porous structures distribution on the surface.
3.5 Photoluminescence analysis
Figure 5a shows the PL excitation and emission spectra. It was observed that the main peaks of excitation when monitoring the UV emission at 395 nm are at 285 and 332 nm. Both absorption bands are attributed to the host absorption [26]. The emission spectra reveal that both undoped and Pb2+-doped ZnAl2O4 powders exhibit UV emission peaks at slightly different positions 390, 395 and 399 nm. Note that within the investigated range of 0–5 mol%, the 1 % Pb2+ had the highest emission intensity. The emission intensity as a function of Pb2+ mol% is presented in Fig. 5b. The Gaussian fit suggests that the optimum dopant concentration of these nanoparticles must be at 1.5 % Pb2+. Above this optimum concentration, the concentration quenching effect on the emission intensity is observed. Similar quenching in luminescence intensity has been observed in TiO2 doped with In- and Ce- by Tang et al. [31] and for TiO2 doped with Pb- by Rahman et al. [32]. Thus, it is strongly believed that doping at higher Pb2+ mol% introduces new defect sites that enhance non-radiative recombination of the excited electrons. Furthermore, the small shift in peak position indicates that the UV emission can be from either the host or Pb2+ ions. The emission from the host at 395 nm is ascribed to be due to the intrinsic intrabandgap defects, such as oxygen vacancies (V *0 ) [4, 26] as shown in Fig. 6b. Da Silva et al. [4] suggested that these defects provide donor levels near the conduction band edge of the oxide.
The luminescence mechanism taking place for all emission arising from the undoped and Pb2+-doped ZnAl2O4 are illustrated in Fig. 6. The electrons in the ground state are excited to the conduction band after the excitation of 285 nm. As far as the host emission is concerned, the excited electrons are de-excited by non-radiative relaxation (NRR) and trapped on the defect states (V *0 ), and then de-excited to the valence band by radiative decay. The incorporation of Pb2+ into the ZnAl2O4 matrix can be interpreted as a development of more new trapping centers [1]. For the emission from the Pb2+, it is proposed here that the mechanism is exactly similar to that one of the host emission but the difference is the trapping centers. As shown in Fig. 5a, the emission from the Pb2+ doped ZnAl2O4 are slightly at different positions, which are at 390 and 399 nm. These shifts of the emissions peaks towards the shorter and higher wavelength suggest that the emission must be from different transitions in Pb2+ ion. Mehnaoui et al. [33] made an attempt to identify the pure electronic transitions between the ground and the excited levels of Pb2+ ion. In their study [33], they were successful to show that Pb2+ ions can occupy two different sites in the apatite and that leads to the probabilities of having two distinct emissions from the lower 3P1 → 1S0 and higher 3P0 → 1S0 energy transitions, which are associated to the Pb2+ ion. It is therefore, reasonable to draw-up the conclusion that these two emission bands from this report must originate from slightly different energy levels transitions from Pb2+ ion as shown in Fig. 6a, c. The emission at the higher wavelength (lower energy), 399 nm, is assign to be from the 3P0 → 1S0 transition [12, 33], which must be within the host bandgap with possibilities of NRR and radiative decay. Finally, the emission from the lower wavelength, 390 nm, (higher energy) is attributed to the 3P1 → 1S0 transition induced by Pb2+ ion. In this case, because of the bandgap energy (~3.18 eV), it is proposed that some of the Pb2+ energy levels are trapped within the conduction band of the host (since the host band gap is 3.14 eV see Fig. 6b). Thus, it is concluded that the Pb2+ doping results in the modification of the bandgap energy and defects levels within the host matrix. As anticipated, the emission from the Pb2+ energy level trapped within the conduction band has the maximum intensity as shown in Fig. 5a. Note that the lifetime and afterglow spectrum were not gathered and therefore they are not discussed in this report.
4 Conclusions
The X-ray diffraction data revealed that the annealed samples consist of highly crystalline cubic ZnAl2O4. Varying the mol% of Pb2+ ions does not affect the crystal structure of the phosphor. The shifting of the 311 reflection into the higher angle is attributed to the decrease in the lattice spacing. The results also suggest that the good particle agglomeration and voids leads to an easy escape routes to the emitted light with the consequent of enhancing the luminescence of a phosphor. The PL results demonstrated that undoped and Pb2+ impurities activated ZnAl2O4 emits at slightly different UV positions. It is concluded that the emission can be from either the host or Pb2+ ions. The incorporation of Pb2+ ions influences the defects levels position and bandgap energy. At the higher Pb2+ mol%, the luminescence quenching behaviour occurs due to the introduction of more new defect sites that enhance non-radiative recombination of the excited electrons.
References
Borse P, Vogel W, Kulkarni S (2006) J Colloid Interface Sci 293:437
Ehlert O, Osvet A, Batentschuk M, Winnacker A, Nann T (2006) J Phys Chem B 110:23175
Zhang D, Wang C, Liu Y, Shi Q, Wang W, Zhai Y (2012) J Lumin 132:1529
Da Silva AA, Goncalves A, Davolos MR (2009) J Sol–Gel Sci Technol 49:101
Ciupina V, Carazeanu I, Prodan G (2004) J Optoelectron Adv Mater 6:1317
Singh V, Chakradhar RPS, Rao J, Kim DK (2008) J Lumin 128:394
Lakshminarayana G, Wondraczek L (2011) J Solid State Chem 184:1931
Nikl M, Novoselov A, Mihokova E, Polak K, Dusek M, McClune B, Yoshikawa A, Fukuda T (2005) J Phys Condens Matter 17:3367
Seltur AA, Srivastava AM (2006) Opt Mater 29:410
Folkerts HF, Hamstra MA, Blasse G (1995) Chem Phys Lett 246:135
Sun Q, Wang J, Shi J (2010) J Solid State Chem 183:1174
Zorenko Y, Gorbenko V, Voznyak T, Zorenko T (2008) Phys Statis Solidi B 245:1618
Bol AA, Meijerink A (2001) Phys Chem 3:2105
Keller JT, Agrawal DK, McKinstry HA (1998) Adv Ceram Mater 3:420
Yuan FL, Hu P, Yin CL, Huang SL, Li JL (2003) J Mater Chem 13:634
Chen Z, Shi E, Zheng Y, Li W, Wu N, Zhong W (2002) Mater Lett 56:601
Adak AK, Pathak A, Pramanik P (1998) J Mater Sci Lett 17:559
Wu Y, Du J, Choy K, Hench LL, Guo J (2005) Thin Solid Films 472:150
Valenzuela MA, Bosch P, Aguilar-Rios G, Montoya A, Schifter I (1997) J Sol–Gel Sci Technol 18:110
Jamal EMA, Kumar DS, Anantharaman (2011) Bull Mater Sci 34:251
Cullity BD (1978) 1956 Elements of X-ray diffraction, 2nd edn. New York, Addison Wesley
Hou Q, Meng F, Sun J (2013) Nanoscale Res Lett 8:144
Zawadzki M (2006) Solid State Sci 8:14
Chen SB, Ma YB, Chen L, Xian K (2010) Geochem J 44:233
Lumbarda VA (2003) Mech Mater 35:53
Da Silva AA, Goncalves A, Davolos MR, Santagneli SH (2008) J Nanosci Nanotechnol 8:5690
Jamal EMA, Kumar DS, Anantharaman (2011) Bull Mater Sci 34:251
Kuang WX, Fan YN, Yao KW, Chen Y (1998) J Solid State Chem 140:354
Bajpai AK, Likhitkar S (2001) Bull Mater Sci 36:15
Zhou Y, Bruening ML, Bergbreiter DE, Crooks RM, Wells M (1996) J Am Chem Soc 118:3773
Tang H, Berger H, Schmid PE, Levy F, Burri G (1993) Solid State Commun 87:847
Rahman MM, Krishna KM, Soga T, Jimbo T, Umeno M (1999) J Phys Chem Solid 60:201
Mehnaoui M, Ternane R, Panczer G, Trabelsi-Ayadi M, GBoulon G (2008) J Phys Condens Matter 20:275227
Acknowledgments
This work is supported by the South African National Research Foundation (NRF) and the research fund of the University of the Free State. The PL system used in this study is supported both technically and financially by the rental pool programme of the National Laser Centre.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Motloung, S.V., Dejene, F.B., Swart, H.C. et al. Effects of Pb2+ ions concentration on the structure and PL intensity of Pb-doped ZnAl2O4 nanocrystals synthesized using sol–gel process. J Sol-Gel Sci Technol 70, 422–427 (2014). https://doi.org/10.1007/s10971-014-3302-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-014-3302-z