Abstract
Pure and silver doped nanoparticles of titanium dioxide (TiO2) was prepared using novel, modified sol–gel method. The samples were characterized by transmission electron microscopy, X-ray diffraction, N2 adsorption measurement, atomic absorption spectroscopy (AAS), UV–vis spectroscopy (UV–vis). The antibacterial activity of the prepared samples was indicated by minimal inhibitory concentrations (MIC) and minimal bactericidal concentrations (MBC) values according to the reference methods of Clinical and Laboratory Standards Institute for the determination of MIC of aerobic bacteria by broth microdilution. The results showed very good antibacterial activity of silver nanoforms to bacteria strains: Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli and Klebsiella pneumoniae. The sensitivity of the tested bacteria to silver nanoforms depends on the crystalline form of the carrier—TiO2, its surface area, porosity, the content of silver, its particle size and oxidation state. The originality of this work is the synthesis of novel type of nanocomposites TiO2 doped with silver and determination its excellent antibacterial activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With regards to its unique photocatalytic activity, titanium dioxide (TiO2) has received considerable attention in recent years. Since 1977, following Frank and Bard [1] confirming the possibility of using TiO2 in the degradation of cyanide in water; there has been an increased interest in the environmental, medical and biological application of TiO2 [1, 2]. Additional research has confirmed its good efficacy in the degradation of a wide variety of organic and inorganic pollutants [3–10]. Photocatalytic oxidation as a technique for microbial disinfection was first demonstrated by Matsanuga et al. [9]. Titanium white is, among other things, used in cosmetics as a white pigment in cream, face powder, lipstick and sunscreen [11]. TiO2 is found in three main crystallographic forms in the environment: anatase, rutile and brookite [12]. The anatase type has been acknowledged as the highest in photocatalytic properties and may be used in various applications, also as a carrier of biologically active particles e.g. silver [13]. There is not much information about biological activity of anatase without UV irradiation which is necessary for photocalatytic activity. There is also no information about biological activity of amorphous TiO2 as a carrier of silver ions or silver nanoparticles. We prepared various forms of TiO2—amorphous and anatase (both doped with silver) and compared its antibacterial activity without UV irradiation.
Silver has been exploited for its medicinal properties for centuries. Its biological activity has been confirmed against Gram-positive and Gram-negative, aerobe and anaerobe bacteria, fungi and protozoan. Chemotherapeutic agents as opposed to antibiotic ones have shown an oligodynamic effect [14–18]. Involving metals by bacteria from culture medium can take place by physical adsorption of ions to the surface of bacteria (van der Waals force) exchanging ions or connections between metal ion and groups of macro and macromolecules, especially in elements such as oxygen, sulfur and nitrogen [19]. Silver is toxic to microorganisms and poisons the respiratory enzymes and components in the microbial electron transport system. Additionally, silver can interact with structural proteins such as fimbriae—protein structures located on the cell’s surface of bacteria, responsible for adhesion to the artificial surface or macroorganism cells. Other research connects silver accumulation in bacterial cells and its interaction with cytosolic proteins, mitochondrial enzymes and DNA or RNA synthesis [20]. Resistance of microorganisms to silver is rare [20, 21]. Bacteria can combat metal ions by decreasing the synthesis of outer membrane proteins, cellular efflux system (active or chemoosmotic) [21] and bound by cells in the form of an intracellular complex [20]. Bacteria can also transform ions to their less toxic form [21, 22]. It confirms very good properties of silver in biological and medical applications as alternative way of killing bacteria.
The originality of this work is the new method of synthesis of very small particles of TiO2 doped with silver as a new type of nanocomposities, which exhibit excellent antimicrobial efficacy without additional factor such as UV irradiation. The tested bacteria strains (Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli and Klebsiella pneumoniae) are responsible for most hospital infections such as: wound infections and urinary track infections. We showed that the titanium dioxide doped silver nanocomposites can be used in numerous biomedical, pharmaceutical and industrial applications also without UV irradiations.
2 Experimental
2.1 Reagents
Titanium (IV) n-butoxide (Ti(O–Bu), 99%), ethyl alcohol (C2H5OH, 96%), methyl alcohol (CH3OH), ammonium hydroxide (NH3 × H2O, 25%), hydrofluoric acid (HF, 40%) and acetone were purchased from POCh. Glucose (C6H12O6), potassium hydroxide (KOH) was bought from Chempur, and the silver nitrate (AgNO3, 99%) was purchased from Aldrich. Deionized water was used in each of the experimental preparations.
2.2 Medium
Muller Hinton Agar (MHA), Muller Hinton Broth (MHB) was purchased from Biocorp.
2.3 Synthesis of amorphous and crystalline TiO2 doped with silver (TiO2:Ag0, TiO2a:Ag0, TiO2:Ag0/Ag+, TiO2a:Ag0/Ag+, TiO2:Ag0/Ag0 and TiO2a:Ag0/Ag0 nanocomposites)
Method synthesis of TiO2 doped with silver (TiO2:Ag0 and TiO2a:Ag0) consists in doping TiO2 with silver during synthesis process.
Titanium dioxide doped with silver—amorphous TiO2:Ag0 and crystalline TiO2a:Ag0—were synthesized through modified sol–gel methods [23]. In our propose silver nanoparticles were incorporated in a TiO2 matrix during the carrier synthesis. In the sol–gel process TiO2 is usually prepared by the following steps: hydrolysis and polycondensation of titanium alkoxides (Ti(OR)n). Sol–gel reaction occurred in acetone environment, in presence of catalyst (ammonium hydroxide) and hydrofluoric acid. Titanium n-butoxide (6 ml) and hydrofluoric acid (250 μl) was added drop wise to acetone (50 ml) with stirring. Next, ammonium hydroxide (2 ml) and diamminesilver (I) ([Ag(NH)2]+) was added. The solution was stirred for 30 min. Preparation of diamminesilver (I) [Ag(NH3)2]+ is described below.
The gel was then washed in methyl alcohol and water and centrifuged at 4,000 rpm. Ready gel was dried at 80°C. In preparing the crystalline form it was calcined at 400°C for 10 h at a ramp rate 5°C/min after drying.
Such prepared samples may be subjected to additional silvered. To prepare TiO2 doped with additional silver (TiO2:Ag0/Ag+, TiO2:Ag0/Ag0, TiO2a:Ag0/Ag+ and TiO2a:Ag0/Ag0) the powder of amorphous and crystalline TiO2 with silver (TiO2:Ag0, TiO2a:Ag0) were treated with diamminesilver (I) [Ag(NH3)2]+ or diamminesilver (I) [Ag(NH3)2]+ and reducing factor (glucose). The process consisted of two steps: impregnation and/or reduction. First, the powder of TiO2:Ag0 and TiO2a:Ag0—0.2 g—was impregnated with a silver solution [Ag(NH3)2]+ at 22°C per 24 h (0.4 M) and distilled water (to final volume 22 ml). The products (TiO2:Ag0/Ag+ or TiO2a:Ag0/Ag+) was then washed with methyl alcohol and water to remove the excess of silver and centrifuged at 4,000 rpm. Next samples with additional silver ions were abandon or were submitted to a reducing chemical factor—glucose (6.8%) to prepared silvered TiO2 with double silver nanoparticles—TiO2:Ag0/Ag0 or TiO2a:Ag0/Ag0 [24, 25]. The entire mixture was stirred for 15 min and stored at 70°C per 24 h. The product was then washed with methyl alcohol and distilled water, centrifuged at 4,000 rpm and suspended in a suitable amount of water. All prepared samples are described in Table 1.
2.4 Preparation of diamminesilver (I)[Ag(NH3)2]+
The diamminesilver (I) nitrate solution (Tollen’s reagent) was prepared by taking 0.1 M of the silver nitrate solution and adding a solution of potassium hydroxide to form a brown precipitate [24, 25]. The sediment was then dissolved in ammonium hydroxide (25%), and stirred until all the precipitate disappeared.
2.5 Characteristics of TiO2:Ag0
The morphology of the samples was investigated by transmission electron microscopy (TEM, Philips CM20 Super Twin). The crystalline phase was identified using a X-ray diffraction (XRD, Stoe). Porosity and Brunauer-Emmett-Teller BET surface area were obtained from nitrogen adsorptions isotherms (Autosorb 1-C, Quantachrome Instruments). The contents of silver were measured using an atomic absorption spectroscopy (AAS—Perkin Elmer 1100).
2.6 Bacteria strains
The following bacteria strains from the American Type Culture Collection were tested:
Staphylococcus aureus ATCC 6538 (Gram-positive bacteria), E. coli ATCC 11229 (Gram-negative bacteria), K. pneumoniae ATCC 4352 (Gram-negative bacteria). Clinical isolates of bacteria (from urinary track infections and wounds) strains were also tested (from species of S. aureus, E. coli and K. pneumoniae).
Before the test the purity of the colonies were verified. For the antibacterial test 16–20 h of bacteria incubation in MHB was used.
2.7 Antibacterial effect of amorphous and crystalline titanium doped with silver (TiO2:Ag0, TiO2a:Ag0, TiO2:Ag0/Ag+, TiO2a:Ag0/Ag+, TiO2:Ag0/Ag0 and TiO2a:Ag0/Ag0 nanospheres)
The antibacterial effect of amorphous and crystalline TiO2:Ag0, TiO2a:Ag0, TiO2:Ag0/Ag+, TiO2a:Ag0/Ag+, TiO2:Ag0/Ag0 and TiO2a:Ag0/Ag0 nanospheres was determined by minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) values according to reference methods of Clinical and Laboratory Standards Institute (CLSI) for the determination of MICs of aerobic bacteria by broth microdilution. The MIC values were the lowest concentration of silver on a microplate that inhibited the growth of bacteria after 16–19 h of incubation at 37°C. MBC had the lowest concentration of the extract in which 99.9% of bacteria were killed [26, 27]. The lower MIC and MBC values the better antibacterial activity. In the experimental procedure, MHB and MHA were used as a medium. Stock of the antimicrobial agent’s dilution was prepared according to the reference method and 200 ml per every spot were poured. Preparation of inoculum: adjusting the 0.5 McFarland standard contains approximately 1–2 × 108 CFU/ml (1) and diluted 0.5 McFarland suspension in sterile saline in the ratio of 1:10 (2). The final inoculum was 104 CFU per spot. Nanoparticles-free spots with medium and cultures were used as growth controls.
3 Results and discussion
3.1 Synthesis and characterization of silvered TiO2
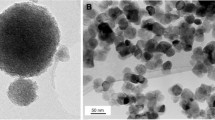
We have found that Ti(O-Bu) hydrolysis and sol–gel condensation in the presence of acetone is much better than in ethanol (data not described) and dropping small concentrations of hydrofluoric acid causes a decrease in the size of TiO2 particles. The particle size of TiO2 depends on composition, calcination temperature and the grain size [28]. Although the radius of silver ion (Ag+) is much larger than that of Ti4+ ion and Ag+ ion introduced by the impregnation process couldn’t enter into the lattice of the anatase phase to form a stable solid solution [29], adding diamminesilver (I) to the prepared mixture of TiO2 produced silver nanoparticles of size less than 5 nm embedded in the structure and on the surface of TiO2. Silver embodied to the TiO2 structure during synthesis (TiO2:Ag0 and TiO2a:Ag0) can also be a source for additional content of silver. It caused increase content of silver from 1.8 to 11.6% (for amorphous TiO2:Ag0) and from 1.6 to 2.2% (for crystalline TiO2a:Ag0). We indicated experimentally that the synthesis time can be reduced from 120 to 30 min, which does not impact the efficacy of technology. Figures 1–3 show TEM images of the prepared basic samples. Figure 1 shows amorphous TiO2:Ag0 and silver nanoparticles on the amorphous TiO2 are presented on Fig. 2. We have indicated that TiO2 size is smaller than 100 nm and particle size of silver embodied to the structure of TiO2 is smaller than 5 nm. Figure 3 shows crystalline TiO2 doped with silver during the synthesis (TiO2a:Ag0). There are seen particles of anatase (with size about 50 nm) and silver nanoparticles with size below 5 nm. Calcination of the amorphous TiO2:Ag0 caused an increase of TiO2 particles size and didn’t change the silver crystallite size. In result the specific surface area of the powder decreased from 424 ± 8 m2/g (for amorphous TiO2:Ag0) to 37.5 ± 0.2 m2/g (for crystalline TiO2a:Ag0) (data shown in Table 2).
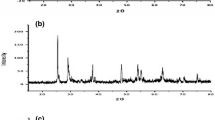
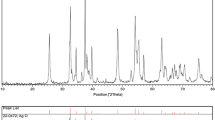
The crystalline character of TiO2 was checked by electron diffraction. The electron diffraction pattern in Fig. 4 shows that the samples are composed of an amorphous or a crystalline form. In the case of the amorphous sample TiO2:Ag0 diffuse rings in diffraction pattern are visible (Fig. 4a). The diffraction pattern of the crystalline sample TiO2a:Ag0 confirms its anatase form (Fig. 4b). Results obtained by electron diffraction coincide with the XRD results. XRD of the amorphous and crystalline TiO2 doped with silver (TiO2:Ag0 and TiO2a:Ag0) are shown on the Fig. 5. All reflections in the diffraction patterns of TiO2 shown in Fig. 5b were identified as belonging to anatase. In the pattern of the amorphous TiO2, no reflections are observed (Fig. 5a). However, similar as in [30], there are no obvious peaks showing the presence of silver in the XRD of the silver doped titania samples (Fig. 5a, b). It probably results from very small size of silver nanoparticles (<5 nm) and its low content (below 2%). In comparison to Chao et al. [29] TiO2a:Ag0 powder calcined already at 400°C is well crystallized.
The room temperature UV–vis diffuse reflectance spectra of amorphous and crystalline samples doped with silver are presented in Fig. 6. It is well known that the UV–vis absorption spectra of the Ag nanoparticles with a size range from 3 to 20 nm exhibit a single absorption peak at 410 nm [6]. As can be seen from curve A in Fig. 6 nanocomposites of crystalline TiO2 have a broad intense absorption below ca. 400 nm. This is the characteristic absorption of TiO2 corresponding to the charge transfer process form the valence band to conduction band in anatase [31]. Lack of visible silver absorption peak in samples TiO2:Ag0 (B) and TiO2a:Ag0 (A) can be due to small size or low concentration of the silver nanoparticles in the TiO2 powder (Fig. 6).
Figure 7 illustrates the N2 adsorption–desorption isotherm of the powders. The isotherm of TiO2:Ag0 powder before annealing is a typical type IV with hysteresis and isotherm of TiO2a:Ag0 powder is typical for mesoporous adsorbent (pore diameter from 2 to 50 nm). Effect of the drying and calcination temperature on the surface area and pore size of the both prepared samples are summarized in Table 2. The surface area of the powders decreased drastically while their pore size increased after calcination at 400°C [32].
3.2 Antibacterial activity
The originality of this work is the synthesis of novel type of TiO2 and determination antibacterial activity its crystalline and non crystalline forms. Results of the MIC and MBC values obtained for all samples are summarized in Tables 3, 4. We tested antimicrobial activity of basic forms—TiO2 doped with silver (TiO2:Ag0 and TiO2a:Ag0) and samples with additional silver count (TiO2:Ag0/Ag+, TiO2:Ag0/Ag0, TiO2a:Ag0/Ag+ and TiO2a:Ag0/Ag0). All samples were tested against Gram-positive and Gram-negative bacteria. The best efficacy indicate basic sample based on anatase TiO2a:Ag0. MIC value of this powder is very low for all bacteria strains: 0.4 μg/ml! However Keleher et. al. [33] in their research obtained MIC values for the crystalline TiO2/Ag0 particles at the level of 6.4 μg/ml for E. coli and 3.9 μg/ml for S. aureus strains. Very good efficacy also indicates a sample with additional silver nanoparticles form (Ag0) of the same samples (TiO2a:Ag0/Ag0): MIC values from 0.4 to 3.2 μg/ml (depending on bacteria strains). It is interesting that antibacterial activity is inversely proportional to surface area (lower in crystalline forms). Results confirm that affecting factor is also silver count and size; the lower count of silver particles, the better antibacterial efficacy (Tables 3, 4). It is also interesting that antibacterial activity dependence on silver oxidation state. In case of amorphous and crystalline TiO2 is better for sample with additional silver ions (TiO2:Ag0/Ag+ and TiO2a:Ag0/Ag+) than additional silver nanoparticles (TiO2:Ag0/Ag0 and TiO2a:Ag0/Ag0). This may be related to the ionization efficacy of Ag+ on the surface of the particles and its ability to be released from the particles and diffuse to the bacterial cell wall. In case of amorphous and crystalline form of TiO2 doped with additional silver ions count (samples signed as TiO2:Ag0/Ag+ and TiO2a:Ag0/Ag+) we have indicated MIC values approach to MBC values. It prove high antibacterial efficacy We suggest that samples, which already has silver present in the form of Ag+, can directly react with cell membranes, whereas the Ag metal bound to the TiO2 particles surface must first dissolution to form Ag+ and then migrate to the bacteria cell. Keleher et al. [33] suggested that the smaller amounts of silver ions are needed to produce the same antibacterial effect as Ag metal.
Thiel et al. [8] suggested that efficient bactericidal behavior of silver nanoparticles results from the amounts of silver: the higher number of silver atoms on the surface, the higher the antibacterial effect. We indicated that the contents of silver have an influence on the antibacterial effect but is inversely proportional to bactericidal efficacy.
Received results indicate differences between microorganisms belonging to two groups. From our test data results that Gram-negative bacteria (tested E. coli and K. pneumoniae) are more sensitive to silver nanoforms than Gram-positive S. aureus. This may result from a difference in the building of the cell-wall. Guillard et al. [7] propose that bacteria which have more fimbriae are more quickly inactivated in the presence of TiO2/Ag0 than those without fimbriae. These protein surface structures help adhere the bacteria cell to TiO2 carriers. It is well known that fimbriae are produced more by Gram-negative bacteria than Gram-positive [34], so we suggest that the higher sensitivity of E. coli and K. pneumoniae results from the presence of fimbriae on the surface of these bacteria.
4 Conclusions
Modified sol–gel technology has made it possible to synthesize nanoparticles of TiO2 with a diameter about 50 nm. Preparation of silver-coated TiO2 is possible by the impregnation and chemical reduction of silver ions during the synthesis of TiO2. An amorphous TiO2 heated at 400°C transformed into crystalline anatase. The surface area of TiO2 decreases while pore size increases during calcination. Sensitivity of tested bacteria strains to silver impregnated TiO2 depends on the crystalline form of carrier, silver particle size, powder surface area, its porosity, the content of silver, and its oxidation state. We have proven that crystalline TiO2 may be very good carrier for silver. Samples synthesized by us may be used as very attractive antimicrobial agents excluding additional factor such as UV irradiation.
References
Frank S, Bard AJ (1977) Heterogenous photocatalytic oxidation of cyanide ion in aqueous solution at TiO2 powder. J Am Chem Soc 99:303–304
Frank SN, Bard AJ (1977) Heterogenous photocatalytic oxidation of cyanide and aqueous solutions at semiconductor powders. J Phys Chem 81:1484–1488
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Fujishima A, Rao TN, Tryk DA (2000) Titanium dioxide photocatalysis. J Photochem Photobiol A Photochem Rev 1:1–21
Alrousan DMA, Dunlop PSM, McMurray TA, Byrne JA (2009) Photocatalytic inactivation of E. coli in surface water using immobilized nanoparticles TiO2 films. Water Res 43:47–54
Akhavan O (2009) Lasting antibacterial activities of Ag-TiO2/Ag/a-TiO2 nanocomposite thin film photocatalyst under solar light irradiation. J Colloid Interface Sci 336:117–124
Guillard Ch, Bui TH, Felix C, Moules V, Lina B, Lejeune P (2008) Microbiological disinfection of water and air by photocatalysis. C.R. Chimie 11:107–113
Thiel J, Pakstis L, Buzby S, Raffi M, Ni C, Pochan DJ (2007) Antibacterial properties of silver-doped titania. Small 5:799–803
Matsunaga T, Tamoda R, Nakajima T, Wake H (1985) Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol Lett 29:211
Dror-Ehre A, Mamane H, Belenkova T, Markovich G, Adin A (2009) Silver nanoparticles-E. coli colloidal interaction in water and effect on E. coli survival. J Colloid Interface Sci 339:521–526
Molski M (2009) Chemia piękna. PWN, Warszawa
Li Y, White T, Lim SH (2003) Structural control and its influence on photoactivity and phase transformation of titania nanoparticles. Ann Rev Mater Sci 5:211–215
Yin HB, Wada Y, Kitamura T, Kambe S, Murasawa S, Mori H et al (2001) Hydrothermal synthesis of nanosized anatase and rutile TiO2 using amorphous phase TiO2. J Mat Chem 11:1694–1703
Bugla-Płoskońska G, Leszkiewicz A, Borak B, Jasiorski M, Drulis-Kawa Z, Baszczuk A et al (2007) Bactericidal properties of silica particles with silver islands located on the surface. Int J Antimicrob Agents 29:738–748
Jasiorski M, Leszkiewicz A, Brzeziński S, Bugla-Płoskońska G, Malinowska G, Borak B (2009) Textile with silver silica spheres: its antimicrobial activity against E. coli and S. aureus. J Sol–Gel Sci Tech 51:330–334
Bugla-Płoskońska G, Jasiorski M, Leszkiewicz A, Borak B, Drulis-Kawa Z, Baszczuk A (2008) Silver nanoislands located on the silica spheres and its antimicrobial activity against K. pneumoniae strain. Nano Sci Nano Tech Indian J 2(2–3):45–47
Bugla-Płoskońska G, Jasiorski M, Leszkiewicz A, Borak B, Baszczuk A, Brzeziński S et al (2008) Bakteriobójcze działanie immobilizowanych preparatów srebra i możliwość ich praktycznego zastosowania. Farm Przegl Nauk 37:23–26
Samuel U, Guggenbichler JP (2004) Prevention of catheter-related infections: the potential of a new nano-silver impregnated catheter. Int J Antimicrob Agents 23S1:75–78
Kojło A, Godlewska-Żyłkiewicz B (2007) Wykorzystanie mikroorganizmów do wydzielania śladów metali w przepływowych technikach analitycznych. Laboratorium-Przegląd Ogólnopolski 6:24–28
Atiyeh BS, Costagliola M, Hayek SN, Dibo SA (2007) Effect of silver on burn wound infection control and healing: review of the literature. Burns 33:139–148
Silver S (2003) Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. Microbiology 27:341–353
Rosen BP (1996) Bacterial resistance to heavy metals and metalloids. J Biol Inorg Chem 1:273–277
Stöber W, Fink A, Bohn E (1968) Controlled growth of monodisperse silica spheres in micro size range. J Colloid Interface Sci 26:62–69
Peterson MSM, Bouwman J, Chen A, Deutsch M (2007) Inorganic metallodielectric materials fabricated using two single step methods based on the Tollen’s process. J Colloid Interface Sci 306:41–49
Wysocka K, Leszkiewicz A, Kowalczyk J, Stręk W, Doroszkiewicz W, Podbielska H (2007) Nanomateriały krzemionkowe domieszkowane srebrem i ich możliwe zastosowania w medycynie. Acta Bio-Opt Inf Med 3(13):22–25
National Committee for Clinical Laboratory Standards (2000) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard—fifth edition M7-A5, 20(2)
Hryniewicz W, Sulikowska A, Szczypa K, Skoczyńska A, Łuczak-Kadłubowska A, Gniatkowski M (2006) Rekomendacje doboru testów do oznaczania wrażliwości bakterii na antybiotyki i chemioterapeutyki. Polish National Institute of Public Heath, Warszawa
Wetchakun N, Phanichphant S (2008) Effect of temperature on the degree of anatase-rutile transformation in titanium dioxide nanoparticles by the modified sol-gel method. Curr App Phys 8:343–346
Chao HE, Yun YU, Xingfang HU, Larbot A (2003) Effect of silver doping on the phase transformation and grain growth of sol–gel titania powder. J Eur Ceram Soc 23:1457–1464
Seery MK, George R, Floris P, Pillai SC (2007) Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J Photochem Photobiol A Chem 189:258–263
Li H, Duan X, Liu G, Liu X (2008) Photochemical synthesis and characterization o Ag/TiO2 nanotube composites. J Mater Sci 43:1669–1676
Mohammadi MR, Fray DJ, Mohammadi A (2008) Sol–gel nanostructured titanium dioxide: controlling the crystal structure, crystallite size, phase transformation, packing and ordering. Micro Meso Mater 112:392–402
Keleher J, Bashant J, Heldt N, Johnson L, Li Y (2002) Photo-catalytic preparation of silver-coated TiO2 particles for antibacterial applications. World J Microbiol Biotechnol 18:133–139
Baj J, Markiewicz Z (2006) Biologia molekularna bakterii. PWN, Warszawa
Acknowledgments
This work was supported by grant Ministry of Science and Higher Education NO. NN405 193136. The authors thank dr Jerzy Kowalczyk for research support and dr Kamila Korzekwa for collection of bacteria strains.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Kedziora, A., Strek, W., Kepinski, L. et al. Synthesis and antibacterial activity of novel titanium dioxide doped with silver. J Sol-Gel Sci Technol 62, 79–86 (2012). https://doi.org/10.1007/s10971-012-2688-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-012-2688-8