Abstract
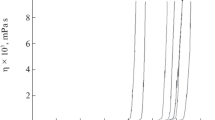
The gelation process of TEOS sols in three different solvents using di-n-butyltin dilaurate (DBTL) as polycondensation catalyst has been investigated. Sol compositions were similar to those employed in the field of stone consolidation for the conservation of historical buildings. Three different systems were studied: TEOS in ethanol (S-EtOH) which was tested to explain gelation in protic solvents; TEOS in a mixture of methylethylketone/acetone (S-MA) to represent aprotic solvents; and TEOS in a blend of MEK/ethanol (S-ME) for comparison of a system with properties intermediate between protic and aprotic solvents. The gelation process was studied by measuring the viscoelastic behavior near the gelation point (GP). A scaling exponent (Δ) was determined for the elastic modulus, G(ω)′ and the viscous modulus, G′′(ω), which both follow the same power law, ωΔ, at GP. The fractal dimension, df, was calculated from the scaling exponent, Δ, for each TEOS-DBTL system. For each type of solvent studied, values of Δ from 0.34 to 0.53 with df of 1.9–2.2 were obtained. The results suggest that DBTL leads to a TEOS polycondensation mechanism similar to that observed for a base-catalyst system. However, the change in df suggests that there is a significant effect of the solvent on aggregation mechanisms of the gelation process. A diffusion limited cluster–cluster aggregation mechanism (DLCCA) was observed when ethanol was used as protic solvent, while a reaction limited cluster–cluster aggregation mechanism (RLCCA) was observed for MEK/acetone (aprotic solvent).





Similar content being viewed by others
References
Iler RK (1979) The Chemistry of Silica. John Wiley & sons, New York, US
Brinker CJ, Scherer GW (1990) Sol–Gel Science. Academic Press INC, San Diego CA, USA
Hench LL, West JK (1990) Chem Rev 90:33–72
Sperling LH (1992) Introduction of Physical Polymer Science, 2a edn. Wiley, USA
Bouchaud E, Delsanti M, Adam M, Daoud M, Durand D (1986) J Physique 47:1273–1277
Woignier T, Phalippou J, Sempere R, Pelous J (1988) J Phys France 49:289–293
Djabourov M, Leblond J, Papon P (1988) J Phys France 49:333–334
de Candia A, Del Gadoa E, Fierro A, Sator N, Conigli A (2005) Physica A 38(2–4):239–248
Jokinen M, Györvary E, Rosenholm JB (1998) J Colld Inter Sci 141:205–216
Martin JE, Adolf D, Wilcoxon JP (1989) Physical Review A 39(3):1325–1332
Barnes HA, Hutton JF, Walters K (2001) An Introduction to Rheology. Elsevier, The Netherlands
Hodgson DF, Amis EJ (1990) Macromolecules 23:2512–2519
Hodgson DF, Amis EJ (1991) J Non-Cryst Solds 131:913–920
Naskar MK (2005) J Materials Science 40:1309–1311
Surivet F, Lam TM, Pascault JP, Tho PQ (1992) Macromolecules 25:4309–4320
Wheeler G (2005) Alkoxysilanes and the Consolidation of Stone. The Getty Conservation Institute, Los Angeles CA, USA
Noll W (1968) Chemistry and Technology of Silicones. Academic press, New York
Van der Weij FW (1980) Macromol Chem 181:2541–2548
Salazar-Hernández C, Zarraga R, Alonso S, Sugita S, Calixto S, Cervantes J (2009) J Sol-Gel Sci Technol 49(3):301–310
Winter HH (1987) Polym Eng Sci 27(22):1698–1702
Payró ER, Llacuna JL (2006) J Non-Cryst Solds 352:2220–2225
Brus J, Kotlik P, Karhan J (1997) Collect Czech Chem Commun 62:442–456
Davies AG (1997) Organotin Chemistry. Wiley-VCH, Germany
Craievich A, Santos DI, Aegerter M, Lours T, Zarzyck J (1988) J Non-Cryst Solds 100:424–428
Woignier T, Phalippou J, Vacher R, Pelous J, Courtens E (1990) J Non-Cryst Solds 121:198–204
Vacher R, Woignier T, Pelous J, Courtens E (1988) Physical Review B 37(11):6500–6505
Méndez-Vivar J (2006) J Sol-Gel Sci Technol 38(2):159–164
Acknowledgments
The authors wish to acknowledge the financial support of the Consejo Nacional de Ciencia y Tecnología (CONACYT-Mexico) through grant 25397.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salazar-Hernández, C., Cervantes, J. & Alonso, S. Viscoelastic characterization of TEOS sols in three different solvents when DBTL is used as polycondensation catalyst. J Sol-Gel Sci Technol 54, 77–82 (2010). https://doi.org/10.1007/s10971-010-2160-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-010-2160-6