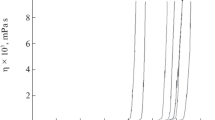
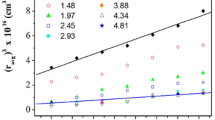
Abstract
Sols composed of dibutyltin dilaureate (DTL) and tetraethyl orthosilicate (TEOS) were prepared using a mixture of methyl ethyl ketone (MEK) and acetone as the solvent in order to study the interaction between the oligomeric Sn and Si species. The hydrolysis molar ratio r (r=nH2O/nM (M: Si, Sn or Si+Sn) was 2. The use of an acid or basic catalyst was avoided, as the sols are intended to be used in the formulation of potential stone consolidants. The sols were studied by several spectroscopic techniques including Small Angle X-ray Scattering (SAXS), 29Si and 119Sn NMR, Fourier Transform Infrared (FTIR) spectroscopy and X-ray diffraction (XRD). According to the spectroscopic results the lauric acid produced by the hydrolysis of DTL modifies the condensation path of the Si species, leading to the formation of two types of oligomeric chains: linear swollen and multiparticle diffusion-limited aggregates, depending on the molar ratio Sn/Si. The 29Si NMR results indicated that the hydrolysis of DTL catalizes the condensation of the Si species, giving as a result higher condensation extents of the Si species in the Sn-Si sols compared to a pure Si sol. Based on the Radial Distribution functions (RDF) and the FTIR results, heterocondensation occurred.
Similar content being viewed by others
References
E.P. Plueddemann, Silane Coupling Agents (Plenum Press, New York, 1982).
C.J. Brinker and G.W. Scherer, Sol-Gel Science, The Physics and Chemistry of Sol-Gel Processing (Academic Press, San Diego, CA, 1990).
L.L. Hench and J.K. West (Eds.), Chemical Processing of Advanced Materials (John Wiley & Sons, Inc., NY, 1992).
L.V. Interrante and M.J. Hampden-Smith (Eds.), Chemistry of Advanced Materials (Wiley-VCH, NY, 1998).
S.J. Blunden, P.A. Cusak, and R. Hill, The Industrial Uses of Tin Chemicals (The Royal Society of Chemistry, London, 1985).
M.J. Hampden-Simith, T.A. Wark, and C.J. Brinker, Coord. Chem. Rev. 112, 81 (1992).
A.G. Davies, Organotin Chemistry (VCH, Germany, 1997).
F.W. Van der Weij, Makromol. Chem. 181, 2541 (1980).
R. Dal Maschio, S. Diré, R. Campostrini, G.D. Soraru, G. Carturan, in Materials Research Society Symposium Proceedings, edited by B.J.J. Zelinski, C.J. Brinker, D.E. Clark, and D.R. Ulrich. (MRS, USA, 1990), vol.180, p. 351.
P.G Harrison, C.C. Perry, D.A. Creaser, X. Li, D.J. Blake, in Chemistry of Advanced Materials (Wiley-VCH, NY, 1998), [4], p. 187.
C.-S. Yang, Q. Liu, S.M. Kauzlarich, and B. Phillips, Chem. Mater. 12, 983 (2000).
O. Glatter, Acta Phys. Austr. 83, 47 (1977).
O. Glatter, J. Appl. Crystallogr. 10, 415 (1977).
H.P. Klug and L.E. Alexander, in X-Ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 2nd edn. (John Wiley & Sons, New York, 1974).
M. Magini and A.J. Cabrini, Appl. Crystallogr. 5(14), 14 (1972).
D.W. Schaefer and K.D. Keefer, in Fractals in Physics edited by L. Pietronero and E. Tosatti (North-Holland, Amsterdam 1986), p. 39–45.
Li Ou and A.B. Seddon, J. Non-Cryst. Solids 210, 192 (1997).
C.J. Pouchert (Ed.), The Aldrich Library of FTIR spectra, 1st edn. (Aldrich Chem. Co., Milwaukee, WI, 1985).
A.B.J. Wojcik and L.C. Klein, J. Sol-Gel Sci. Tech. 4(57), 57 (1995).
M. Pauthé, J. Phalipou, R. Corriu, D. Leclercq, and A. Vioux, J. Non-Cryst. Solids 113, 23 (1989).
Nakamoto, in Infrared and Raman Spectra of Inorganic and Coordination Compounds (Wiley, New York, 1978).
J.M. Brown, A.C. Chaoman, R. Harper, D.J. Mowthorpe, A.G. Davies, and P.J. Smith, J. Chem. Soc., Dalton Trans. 338 (1972).
J. Méndez-Vivar, R. Mendoza-Serna, and L. Valdez-Castro, J. Non-Cryst. Solids 288, 200 (2001).
G. Socrates, Infrared Characteristic Group Frequencies (Wiley, UK, 1980).
E.S. Goins, Alkoxysilane Consolidants: The Effect of the Stone Substrate on the Polymerization Process. PhD Thesis (University College, London, 1995), p. 166.
D. Lien-Vien, N.B. Colthup, W.G. Fateley, and J.G. Graselli, The Handbook of Infrared and Raman Characteristic Frequencies of Inorganic Molecules (Academic Press, USA, 1991).
Joint Committee of Powder Diffraction Standard (JCPDS) files.
S. Prabakar, R.A. Assink, N.K. Raman, C.J. Brinker, in Materials Research Society Symposium Proceedings edited by A.K. Cheetham, C.J. Cheetham, M.L. Brinker, C. Mecartney, Sanchez, MRS Pittsburgh PA, 1994), vol. 346, p. 979.
L. Pauling, The Nature of Chemical Bond (Cornell University Press, NY, 1960).
E.Bosch, et al., US Patent 3,955,988, May 11, 1976.
G. Wheeler, Alkoxysilanes and the Consolidation of Stone (The Getty Conservation Institute, Los Angeles CA, USA 2005), p. 58.
C.A. Grissom, Art and Archaeology Technical Abstracts 18(1), 150 (1981).
G.G. Amoroso and V. Fascina, Stone Decay and Conservation (Elsevier, New York, 1983).
G. Wheeler, J. Dinsmore, L.J. Ransick, A.E. Charola, and R.J. Koestler, Studies in Conservation 29(1), 42 (1984)
S.Z. Lewin and G. Wheeler, in Proc. Fifth International Congress on the Deterioration and Conservation of Stone, edited by G. Felix, and V. Furlan, Laussane (Presses Polytechniques Romandes, 1985) p. 831.
S.M. Bradley, Geological Curator 4(7), 427 (1986).
A.E. Charola and R.J. Koestler, Scanning Electron Microscopy 2, 479 (1986).
G. Scherer and G. Wheeler, in Fourth International Symposium on the Conservation of Monuments in the Mediterranean Basin edited by A. Moropoulou, F. Zezza, E. Kollias, I. Papachristodoulou, (Technical Chamber of Greece, Athens, 1997), vol.3, p. 355.
J. Cervantes, G. Mendoza-Díaz, D.E. Alvarez-Gasca, A. Martínez-Richa, Solid State Nuclear Magnetic Resonance 13(4), 263 (1999).
G. Wheeler, J. Méndez-Vivar, E.S. Goins, S.A. Fleming, C.J. Brinker, in Proc. Ninth International Congress on Deterioration and Conservation of Stone edited by V. Fassina, Venice (Elsevier, 2000), p. 541.
A.B. Oliver, APT Bulletin 33(2/3), 39 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Méndez-Vivar, J. The interaction of dibutyltin dilaureate with tetraethyl orthosilicate in sol-gel systems. J Sol-Gel Sci Technol 38, 159–166 (2006). https://doi.org/10.1007/s10971-006-6351-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-006-6351-0