Abstract
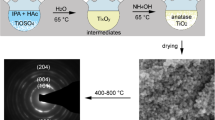
Ti(OPri)4 reacts with HOSi(OtBu)3 in anhydrous benzene in 1:1 and 1:2 molar ratios to afford alkoxy titanosiloxane precursors, [Ti(OPri)3{OSi(OtBu)3}] (A) and [Ti(OPri)2{OSi(OtBu)3}2] (B), respectively. Further reactions of (A) or (B) with glycols in 1:1 molar ratio afforded six complexes of the types [Ti(OPri)(O–G–O){OSi(OtBu)3}] (1A–3A) and [Ti(O–G–O){OSi(OtBu)3}2] (1B–3B), respectively [where G = (CH2)2 (1A, 1B); (CH2)3 (2A, 2B) and {CH2CH2CH(CH3)} (3A, 3B)]. Both (A) and (B) are liquids while all the other products are viscous liquids which get solidified on ageing. Cryoscopic molecular weight measurements of the fresh products indicate their monomeric nature. FAB mass studies of (A) and (B) also indicate monomeric nature. However, FAB mass spectra of the two representative solids (1A) and (2B) suggest dimeric behavior of the glycolato derivatives. (A) distills at 85 °C/5 mm while other products get decomposed even under reduced pressure. TG analyses of (A), (B), (1A), and (1B) suggest formation of titania–silica materials at 200 °C for (A) and (B) and 350 °C for (1A) and (1B). The products have been characterized by elemental analyses, FTIR and 1H, 13C & 29Si-NMR techniques. All these products are soluble in common organic solvents indicating a homogenous distribution of the components on the molecular scale. The Si/Ti ratio of the oxide may be controlled easily by the composition of the starting precursors. Hydrolysis of the glycol modified derivative, (1A) by the Sol–Gel technique affords the desired homogenous titania–silica material, TiO2·SiO2 in nano-size while, the precursor (A) yields a non-stiochiometric silica doped titania material. However, pyrolysis of (A) yields nano-sized crystallites of TiO2·SiO2. All these materials were characterized by FTIR, powder XRD patterns, SEM images, and EDX analyses.






Similar content being viewed by others
References
Kessler VG, Spijksma GI, Seisenbaeva GA, Håkansson S, Blank DHA, Bouwmeester HJM (2006) J Sol Gel Sci Technol 40:163. doi:10.1007/s10971-006-9209-6
Sanchez C, Julian B, Belleville P, Popall M (2005) J Mater Chem 15:35
Sharma N, Sharma V, Bohra R, Raju VS, Lorenz IP, Krinninger C, Mayer P (2007) Inorg Chim Acta 360:3002. doi:10.1016/j.ica.2007.02.048
Schubert U (2007) Acc Chem Res 40:730. doi:10.1021/ar600036x
Dhayal V, Bohra R, Nagar M, Kaushik A, Mathur S, Barth S (2008) Appl Organomet Chem 22:629. doi:10.1002/aoc.1448
Swamy KCK, Chandrasekhar V, Harland JJ, Holmes JM, Day RO, Holmes RR (1990) J Am Chem Soc 112:2341. doi:10.1021/ja00162a039
Veith M, Rammo A (1996) J Organomet Chem 521:429. doi:10.1016/0022-328X(96)06297-3
Forter KC, Bigi JP, Brown SN (2005) Inorg Chem 44:2803. doi:10.1021/ic048403d
Lickiss PD (1995) Adv Inorg Chem 42:147. doi:10.1016/S0898-8838(08)60053-7
Beckmann J, Dakternieks D, Duthie A, Larchin ML, Tiekink ERT (2003) Appl Organomet Chem 17:52. doi:10.1002/aoc.380
Walawalkar MG, Murugavel R, Roesky HW (1996) In: Corriu R, Jutzi P (eds) Talior made silicon oxygen compounds—from molecules to materials. Vieweg, Braunschweig, p 61
Davis P, Murugavel R (2005) Synth React Inorg Met-Org Nano-Met Chem 35:591. doi:10.1080/15533170500225540
Terry KW, Tilley TD (1991) Chem Mater 3:1001. doi:10.1021/cm00018a008
Terry KW, Lugmair CG, Tilley TD (1997) J Am Chem Soc 119:9745. doi:10.1021/ja971405v
Lugmair CG, Tilley TD (1998) Inorg Chem 37:764. doi:10.1021/ic971211g
Hoebbel D, Nacken M, Schmidt H, Huch V, Veith M (1998) J Mater Chem 8:171. doi:10.1039/a702644g
Terry KW, Su K, Tilley TD, Rheingold AL (1998) Polyhedron 17:891. doi:10.1016/S0277-5387(97)00260-X
Murugavel R, Davis P, Shete VS (2003) Inorg Chem 42:4696. doi:10.1021/ic034317m
Fujdala KL, Tilley TD (2004) Chem Mater 16:1035. doi:10.1021/cm030563k
Coles MP, Lugmair CG, Terry KW, Tilley TD (2000) Chem Mater 12:122. doi:10.1021/cm990444y
Laha SC, Kumar R (2002) J Catal 208:339. doi:10.1006/jcat.2002.3582
Wang XS, Guo XW, Li G (2002) Catal Today 74:65. doi:10.1016/S0920-5861(01)00531-4
Klein S, Thorimbert S, Maier WF (1996) J Catal 163:476. doi:10.1006/jcat.1996.0349
Hutter R, Mallat T, Dutoit D, Baiker A (1996) Top Catal 3:421. doi:10.1007/BF02113865
Jung M (2000) J Sol Gel Sci Technol 19:563. doi:10.1023/A:1008748924836
Livage C, Safari A, Klein LC (2006) J Sol Gel Sci Technol 2:605. doi:10.1007/BF00486318
Yamamoto O, Sasamat T (1992) J Mater Res 7:2488. doi:10.1557/JMR.1992.2488
Singh A, Mehrotra RC (2004) Coord Chem Rev 248:101. doi:10.1016/j.cct.2003.09.004
Boyel TJ (1951) Polym Sci 7:591. doi:10.1002/pol.1951.120070603
Bradley DC, Hancock DC, Wardlaw W (1952) J Chem Soc 2773. doi:10.1039/jr9520002773
Bradley DC, Abd-El-Halim FM, Mehrotra RC, Wardlaw W (1952) J Chem Soc 4609. doi:10.1039/jr9520004609
Wang D, Yu R, Kumada N, Kinomura N (1999) Chem Mater 11:2008. doi:10.1021/cm980579o
Pathak M, Bohra R, Mehrotra RC, Lorenz I-P, Piotrowski H (2003) Trans Met Chem 28:187. doi:10.1023/A:1022901918955
Warren BE (1990) X-ray diffraction, vol 13. Dover Publication, New York
Miller JB, Johnston ST, Ko EI (1994) J Catal 150:311. doi:10.1006/jcat.1994.1349
Andrianainarivelo M, Corriu R, Leclercq D, Mutin PH, Vioux AJ (1996) J Mater Chem 6:1665. doi:10.1039/jm9960601665
Acknowledgments
We are thankful to CSIR and DST-New Delhi for financial support. We thank CSMCRI, Bhavnagar for TGA and IIT, Roorkee for SEM coupled EDX analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dhayal, V., Atal, M.K., Choudhary, B.L. et al. Glycol modified titanosiloxane as molecular precursor for homogenous titania–silica material: synthesis and characterization. J Sol-Gel Sci Technol 52, 97–108 (2009). https://doi.org/10.1007/s10971-009-2008-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-009-2008-0