Abstract
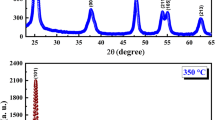
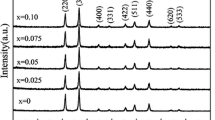
TiO2 nanopowders were produced by sol–gel technique under different synthesis conditions. XRD results have shown that obtained nanopowders are in anatase phase, with the presence of a small amount of highly disordered brookite phase, whereas nanocrystallite size and amount of brookite slightly depend on sol–gel synthesis conditions. Raman measurements confirm these results. The analyses of the shift and width of the most intensive anatase E g Raman mode by phonon confinement model suggest that anatase crystallite size should be in the range between 11 and 15 nm, what is in excellent correlation with XRD results. Obtained results have shown that Raman spectroscopy is a highly sensitive method for the estimation of anatase crystallite size as well as brookite content in TiO2 nanopowders synthesized by variable sol–gel synthesis conditions.









Similar content being viewed by others
Abbreviations
- XRD:
-
X-ray diffraction
- PCM:
-
Phonon confinement model
- JCPDS:
-
Joint committee on powder diffraction standards
References
Venz PA, Kloprogge JT, Frost RL (2000) Langmuir 16:4962. doi:10.1021/la990830u
Murugan AV, Samuel V, Ravi V (2006) Mater Lett 60:479. doi:10.1016/j.matlet.2005.09.017
Sakka S (2006) J Sol-Gel Sci Technol 37:135. doi:10.1007/s10971-006-6433-z
Hart JN, Menzies D, Cheng Y-B, Simon GP, Spiccia L (2006) J Sol-Gel Sci Technol 40:45. doi:10.1007/s10971-006-8387-6
Kim I-D, Rothschild A, Yang D-J, Tuller HL (2008) Sens Actuators B Chem 130:9. doi:10.1016/j.snb.2007.07.092
Granqvist CG, Azens A, Isidorsson J, Kharrazi M, Kullman L, Lindström T, Niklasson GA, Ribbing C-G, Rönnow D, Strømme Mattsson M, Veszelei M (1997) J Non-Cryst Solids 218:273. doi:10.1016/S0022-3093(97)00145-2
Zhang J, Wang X, Zheng W-T, Kong X-G, Sun Y-J, Wang X (2007) Mater Lett 61:1658. doi:10.1016/j.matlet.2006.07.093
Venkatachalam N, Palanichamy M, Arabindoo B, Murugesan V (2007) Mater Chem Phys 104:454. doi:10.1016/j.matchemphys.2007.04.003
Gouadec G, Colomban P (2007) Prog Cryst Growth Char Mater 53:1
Zhang WF, He YL, Zhang MS, Yin Z, Chen Q (2000) J Phys D Appl Phys 33:912. doi:10.1088/0022-3727/33/8/305
Du YL, Deng Y, Zhang MS (2006) J Phys Chem Solids 67:2405. doi:10.1016/j.jpcs.2006.06.020
Gao K (2007) Phys B 398:33. doi:10.1016/j.physb.2007.04.013
Zhang J, Li MJ, Feng ZC, Chen J, Li C (2006) J Phys Chem B 110:927. doi:10.1021/jp0552473
Rodriguez-Carvajal J (1998) FullProf computer program. ftp://charybde.saclay.cea.fr/pub/divers/fullprof.98/windows/winfp98.zip
Kremenovic A, Blanusa J, Antic B, Colomban P, Kahlenberg V, Jovalekic C, Dukic J (2007) Nanotechnology 18:145616
Lakshmi BB, Dorhout PK, Martin CR (1996) Chem Mater 9:857. doi:10.1021/cm9605577
Zhang W, Chen S, Yu S, Yin Y (2007) J Cryst Growth 308:122. doi:10.1016/j.jcrysgro.2007.07.053
Aruna ST, Tirosh S, Zaban A (2000) J Mater Chem 10:2388. doi:10.1039/b001718n
Sun J, Gao L (2002) J Am Ceram Soc 85:2382. doi:10.1111/j.1151-2916.2002.tb00467.x
Díaz-Díez MÁ, Macías-García A, Silvero G, Gordillo R, Caruso R (2003) Ceram Int 29:471. doi:10.1016/S0272-8842(02)00189-X
Venz PA, Frost RL, Kloprogge JT (2000) J Non-Cryst Solids 276:95. doi:10.1016/S0022-3093(00)00267-2
Wang P, Wang D, Xie T, Li H, Yang M, Wei X (2008) Mater Chem Phys 109:181. doi:10.1016/j.matchemphys.2007.11.019
He D, Lin F (2007) Mater Lett 61:3385. doi:10.1016/j.matlet.2006.11.075
Hari-Bala, Guo Y, Zhao X, Zhao J, Fu W, Ding X, Jiang Y, Yu K, Lv X, Wang Z (2006) Mater Lett 60:494. doi:10.1016/j.matlet.2005.09.030
Liu AR, Wang SM, Zhao YR, Zheng Z (2006) Mater Chem Phys 99:131. doi:10.1016/j.matchemphys.2005.10.003
Sugimoto T, Zhou X, Muramatsu A (2003) J Colloid Interface Sci 259:43. doi:10.1016/S0021-9797(03)00036-5
Ohsaka T, Izumi F, Fujiki Y (1978) J Raman Spectrosc 7:321. doi:10.1002/jrs.1250070606
Šćepanović MJ, Grujić-Brojčin MU, Dohčević-Mitrović ZD, Popović ZV (2006) Mater Sci Forum 518:101
Šćepanović MJ, Grujić-Brojčin M, Dohčević-Mitrović Z, Popović ZV (2007) Appl Phys A 86:365. doi:10.1007/s00339-006-3775-x
Li Bassi A, Cattaneo D, Russo V, Bottani CE, Barborini E, Mazza T, Piseri P, Milani P, Emst FO, Wegner K, Pratsinis SE (2005) J Appl Phys 98:074305. doi:10.1063/1.2061894
Kelly S, Pollak FH, Tomkiewicz M (1997) J Phys Chem B 101:2730. doi:10.1021/jp962747a
Bersani D, Lottici PP (1998) Appl Phys Lett 72:73. doi:10.1063/1.120648
Richter H, Wang ZP, Ley L (1981) Solid State Commun 39:625. doi:10.1016/0038-1098(81)90337-9
Campbell IH, Fauchet PM (1984) Solid State Commun 58:739. doi:10.1016/0038-1098(86)90513-2
Spanier JE, Robinson RD, Zhang F, Chan SW, Herman IP (2001) Phys Rev B 64:245407. doi:10.1103/PhysRevB.64.245407
Parker JC, Siegel RW (1990) Appl Phys Lett 57:943. doi:10.1063/1.104274
Zhu KR, Zhang MS, Chen Q, Yin Z (2005) Phys Lett A 340:220. doi:10.1016/j.physleta.2005.04.008
Mikami M, Nakamura S, Kitao O, Arakawa H (2002) Phys Rev B 66:155213. doi:10.1103/PhysRevB.66.155213
Ivanda M, Tonejc AM, Djerdj I, Gotić M, Musić S, Mariotto G, Montagna M (2002) Nanoscale spectroscopy and its applications in semiconductor research, Lecture Notes in Physics, vol 588. Springer, Berlin, p 24
Djaoued Y, Brüning R, Bersani D, Lottici PP, Badilescu S (2004) Mater Lett 58:2618. doi:10.1016/j.matlet.2004.03.034
Yin S, Ihara K, Liu B, Wang Y, Li R, Sato T (2007) Phys Scr T 129:268. doi:10.1088/0031-8949/2007/T129/060
Bersani D, Lottici PP, Lopez T, Ding X-Z (1998) J Sol-Gel Sci Technol 13:849. doi:10.1023/A:1008602718987
Ovenstone J, Yanagisawa K (1999) Chem Mater 11:2770. doi:10.1021/cm990172z
Deshpande SB, Potdar HS, Khollam YB, Patil KR, Pasricha R, Jacob NE (2006) Mater Chem Phys 97:207. doi:10.1016/j.matchemphys.2005.02.014
Khanna PK, Singh N, Charan S (2007) Mater Lett 61:4725. doi:10.1016/j.matlet.2007.03.064
Khan R, Woo Kim S, Kim T-J, Nam C-M (2008) Mater Chem Phys 112:167
Pottier A, Chanéac C, Tronc E, Mazerolles L, Jolivet J-P (2001) J Mater Chem 11:116. doi:10.1039/b100435m
Acknowledgement
Authors express thanks to Mirjana Grujić-Brojčin for the original software solutions which enabled the application of the PCM for numerical simulation of Raman spectra of investigated samples. Authors are also grateful to Toma Radić and Marko Radović for their help during AFM measurements. This work is supported by the Serbian Ministry of Science under project no. 141047, the OPSA-026283 project within the AC FP6 programme and SASA project F-134.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Golubović, A., Šćepanović, M., Kremenović, A. et al. Raman study of the variation in anatase structure of TiO2 nanopowders due to the changes of sol–gel synthesis conditions. J Sol-Gel Sci Technol 49, 311–319 (2009). https://doi.org/10.1007/s10971-008-1872-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-008-1872-3