Abstract
The N 1-methyladenosine residue at position 58 of tRNA is found in the three domains of life, and contributes to the stability of the three-dimensional L-shaped tRNA structure. In thermophilic bacteria, this modification is important for thermal adaptation, and is catalyzed by the tRNA m1A58 methyltransferase TrmI, using S-adenosyl-l-methionine (AdoMet) as the methyl donor. We present the 2.2 Å crystal structure of TrmI from the extremely thermophilic bacterium Aquifex aeolicus, in complex with AdoMet. There are four molecules per asymmetric unit, and they form a tetramer. Based on a comparison of the AdoMet binding mode of A. aeolicus TrmI to those of the Thermus thermophilus and Pyrococcus abyssi TrmIs, we discuss their similarities and differences. Although the binding modes to the N6 amino group of the adenine moiety of AdoMet are similar, using the side chains of acidic residues as well as hydrogen bonds, the positions of the amino acid residues involved in binding are diverse among the TrmIs from A. aeolicus, T. thermophilus, and P. abyssi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Posttranscriptional modifications alter the characteristics of tRNAs in various manners, to fine-tune their functions. The modified nucleoside N 1-methyladenosine is found at four positions: position 9 of mammalian mitochondrial tRNAs, position 14 of mammalian cytoplasmic tRNAPhe, position 22 of tRNA in some bacteria, and position 58 of tRNA in the three domains of life [1]. The N 1-methylation of adenosine abrogates its ability to form a standard Watson–Crick base pair, as also found with m1G, m1I, m3C, m3U, and m3Ψ. Indeed, reverse transcriptases read m1A with very low efficiency, and those in the HIV-1 and Molony murine leukemia viruses utilize the host’s tRNA bearing m1A for their replication [2–5].
In the absence of the m1A9 modification, mammalian mitochondrial tRNALys could adopt an extended hairpin structure that is unproductive in translation, since an undesired base pair between A9 and U64 is tolerated [6, 7]. In yeast, the strain with a defective m1A58 modification is nonviable, because the initiator tRNAMet is degraded [8]. In the native yeast tRNA, the m1A58 of the initiator tRNAMet forms the reverse Hoogsteen base pair with A54, which increases the stability of the three-dimensional structure, while the m1A58 in the other 19 tRNAs forms the reverse Hoogsteen base pair with T54 [9–11]. In the thermophilic bacterium Thermus thermophilus, inactivation of the trmI gene results in a thermosensitive phenotype, suggesting that the m1A58 modification is important for both thermal adaptation and tRNA stability [12]. The m1A58 residue was analyzed by NMR and IR spectral studies, which considered the 1H, 13C, and 15N chemical shifts, the consistency of the sugar pucker and glycosidic conformations with those of the X-ray structure, and the character of the bond between the C6 and N6 atoms [13, 14]. Based on the results, the m1A58 residue in the native tRNA was deduced to be fully protonated, with its charge probably dislocalized from the quaternary N1 atom toward the C6, C5, and C4 atoms. The protonated state of the m1A58 residue is characteristic of the Mg2+-bound native state, and the partial charge in the tRNA elbow region may affect its interaction with the translational machinery [13, 14]. Therefore, the m1A58 modification of tRNA is important for stabilizing the L-shaped structure and for efficient translation [15].
The methyl group of m1A58 is transferred from the methyl donor S-adenosyl-l-methionine (AdoMet) by the TrmI homotetramer in bacteria and archaea, and by the Trm6/Trm61 α2/β2 heterotetramer complex in eukaryotes [8, 12]. The coordinated structural genomics projects on proteins from Mycobacterium tuberculosis determined the first structure of TrmI, as the conserved hypothetical methyltransferase Rv2118c [16]. At the same time, an in silico fold prediction study was reported [17]. Subsequently, the crystal structure of the catalytic domain (residues 70–250) of the TrmI tetramer from Pyrococcus abyssi revealed its mechanism of thermal stabilization, using intersubunit disulfide bonds [18]. The crystal structure of TrmI from T. thermophilus [19] was determined and complemented by biophysical characterizations, which revealed the tRNA binding stoichiometry per TrmI tetramer [19]. The crystal structure of full-length TrmI from P. abyssi was reported with further biochemical characterization of the region specificities [20]. Presently, eight PDB datasets from six species are available, and their structural architectures have been compared [21]. Comprehensive structural genomics projects on a specific organism, typified by that on M. tuberculosis [22], have provided the structural basis to characterize the biological functions of the proteome, including conserved proteins with unknown functions. On the other hand, comparative analyses of large numbers of orthologous and homologous structures, including some acquired by high-throughput capability and successful structural genomics [23–25], will lead to further understanding of the structure–function relationships of proteins and facilitate applications, including protein engineering and drug design. Here, we report the crystal structure of TrmI from Aquifex aeolicus in the complex with AdoMet, determined at 2.2 Å resolution. The overall tetrameric architecture is quite similar to the structures of TrmIs from other species [21]. We examined the similarities and differences in the AdoMet recognition by A. aeolicus TrmI, as compared to those by the TrmIs from T. thermophilus and P. abyssi.
Materials and methods
Cloning, expression, and purification of A. aeolicus TrmI
The aq_311 gene, encoding the A. aeolicus TrmI protein (gi: 15605836) comprising 248 residues, was amplified by PCR using A. aeolicus VF5 genomic DNA and cloned into the pET-21a expression vector (Merck Novagen, Darmstadt, Germany). The expression vector was transformed into the E. coli Rosetta™ (DE3) strain (Merck Novagen). The cells were cultured at 37 °C in LB medium, supplemented with 30 µg/ml chloramphenicol and 50 µg/ml ampicillin. The protein expression was induced by 0.5 mM IPTG. Following an overnight incubation, the cells were harvested by centrifugation and stored at −80 °C. The cells were resuspended in 20 mM Tris–HCl buffer (pH 8.0), containing 300 mM NaCl, 5 mM MgCl2, 0.5 mM EDTA, and 1 mM DTT, and were lysed by sonication on ice. The cell lysate was heat-treated at 70 °C for 30 min to denature most of the E. coli proteins, and was centrifuged at 15,000×g for 20 min at 4 °C. The supernatant was desalted by dialysis against 20 mM Tris–HCl buffer (pH 8.0) containing 1 mM DTT, and applied to a HiTrap Q column (GE Healthcare Biosciences), equilibrated with the same buffer. The protein was eluted with a linear gradient (0–1.0 M) of NaCl, and the target fractions, which eluted around 0.4 M NaCl, were collected. Ammonium sulfate was added to the sample, which was applied to a Resource PHE column (GE Healthcare Biosciences), equilibrated with 20 mM Tris–HCl buffer (pH 8.0) containing 1.2 M ammonium sulfate and 1 mM DTT, and was eluted with a decreasing linear (1.2–0 M) gradient of ammonium sulfate. The target fractions, which were eluted in 0.6–0.3 M ammonium sulfate, were collected and desalted by dialysis. The sample was applied to a Mono S column (GE Healthcare Biosciences), equilibrated with 20 mM Tris–HCl buffer (pH 8.0) containing 1 mM DTT, and was eluted by a linear (0–1.0 M) gradient of NaCl. The fraction that eluted at 0.3 M was concentrated and applied to a HiLoad 16/60 Superdex 75 pg column (GE Healthcare Biosciences), equilibrated with 20 mM Tris–HCl buffer (pH 8.0) containing 150 mM NaCl and 1 mM DTT. The gel filtration elution profile showed one peak at 50 ml, which corresponds to 0.41 column volumes. The protein sample was concentrated to 15 mg/ml by ultrafiltration. The protein purification was analyzed by SDS-PAGE. The electrophoretic mobility of A. aeolicus TrmI is almost the same as that of a marker (29 kDa), in agreement with its theoretical molecular weight (28.7 kDa). The final yield was 2.2 mg/l of culture.
Crystallization and data collection
The A. aeolicus TrmI protein at 10–12 mg/ml concentrations, in 20 mM Tris–HCl buffer (pH 8.0) containing 150 mM NaCl, 1 mM DTT, and 2 mM AdoMet, was used for crystallization. Initial crystallization screening was performed in 1:1 sitting-drop vapor-diffusion reactions at 20 °C, by mixing 1 μl protein solution with 1 μl reservoir solution. The crystals were grown in 0.1 M Tris–HCl buffer (pH 8.4) and 20 % ethanol. The crystals were transferred to 0.1 M Tris–HCl buffer (pH 8.4), 20 % ethanol, and 35 % ethylene glycol for cryoprotection, prior to flash-cooling in liquid nitrogen for data collection. The native dataset was collected on beamline BL41XU at SPring-8 (Table 1). Data collected from a single crystal at 100 K were processed with the HKL2000 program [26].
Structure solution and refinement
The phase was determined by the molecular replacement method, using the coordinates of TrmI from Thermotoga maritima (PDB ID: 1O54) as the starting model, with the program MOLREP [27]. The model was completed using iterative cycles of manual rebuilding in Coot [28] and computational refinement at 2.2 Å in Refmac5 [29] (Table 1).
Structure validation and deposition
The structure validation of the model is summarized in Table 1. The atomic coordinates and structure factors have been deposited in the Protein Data Bank, under the accession code 2YVL.
Sedimentation velocity ultracentrifugation analysis
The A. aeolicus TrmI protein, at a 1 mg/ml concentration in 20 mM Tris–HCl buffer (pH 8.0) containing 150 mM NaCl and 1 mM DTT, was analyzed by ultracentrifugation at 20 °C, in a ProteomeLab XL-I ultracentrifuge (Beckman Coulter) with the An-60 Ti analytical rotor. The sample was ultracentrifuged at 40,000 rpm, and the absorbance at 280 nm was measured. The data were analyzed and the distribution c(M) was calculated by Sedfit [30].
Results and discussion
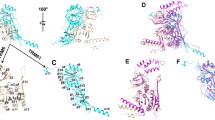
The crystal structure of A. aeolicus TrmI was determined at 2.2 Å resolution by the molecular replacement method, and was refined to R work and R free factors of 19.6 and 23.0 %, respectively (Table 1). The asymmetric unit contains four protomers (A–D) (Fig. 1a) and four AdoMet molecules. The electron density was interpretable for 247 residues (Asn2–Thr248). The A. aeolicus TrmI protomer (Fig. 1b) consists of the small N-terminal domain (residues 2–58) and the C-terminal methyltransferase domain (residues 72–248), which are connected by an α-helical linker (residues 59–71). The N-terminal domain forms a small β sandwich (Fig. 1b), in which the β sheet β2–β1–β6–β5 stacks on the β hairpin β3–β4, along with the small 310-helix η1. The C-terminal domain adopts the typical type I methyltransferase fold, with a central seven-stranded β sheet with the topology β9–β8–β7–β10–β11–β14–β12, flanked by α helices on both sides (Fig. 1b). As reported previously [19], the long β strand β12, in which the head interacts with β13, is characteristic of TrmI among the type I methyltransferases, and it provides a surface for tetramerization.
Crystal structure of A. aeolicus TrmI in complex with AdoMet. a The tetrameric structure of A. aeolicus TrmI. The four protomers are colored pink, cyan, purple, and green. b Protomer structure of A. aeolicus TrmI. The N-terminal domain, the linker helix, and the C-terminal domain are colored pink, purple, and cyan, respectively. The secondary structures are labeled. c The calculated distributions c(M) by Sedfit [30]. d–f Ball-and-stick representations of AdoMet binding by A. aeolicus TrmI (chain A) (d), T. thermophilus TrmI [19] (chain A) (e), and P. abyssi TrmI [20] (chain A) (f). The three amino acid residues surrounding the N6 amino group of AdoMet are labeled with orange rectangles. Hydrogen bonds are depicted by dotted lines with their distances (Å). The figures were created using CueMol (http://cuemol.sourceforge.jp/en/)
We analyzed the oligomeric state of A. aeolicus TrmI in solution by sedimentation velocity ultracentrifugation. The gel filtration elution profile of A. aeolicus TrmI showed one peak between the IgG (158 kDa) and human albumin (66 kDa) markers. Since the theoretical molecular weight of A. aeolicus TrmI is 28.7 kDa, TrmI is suggested to exist as tetramer (114.8 kDa) in solution. The ultracentrifugation analysis, using 1 mg/ml A. aeolicus TrmI, showed one peak at 110 kDa (Fig. 1c), which confirmed that it is tetrameric in solution.
The methyl donor AdoMet is bound in the C-terminal domain of the protein (Fig. 1b). A. aeolicus TrmI recognizes AdoMet by hydrogen bonds from its main-chain and side-chain atoms as well as water-mediated hydrogen bonds (Fig. 1d), in a similar manner to T. thermophilus TrmI (Fig. 1e) [19] and P. abyssi TrmI (Fig. 1f) [20]. The N1 atom of the adenine moiety hydrogen bonds with the main chain amide nitrogen of Phe149 (3.0 Å) of A. aeolicus TrmI. The N6 amino group hydrogen bonds with the side chains of Asp148 (2.9 Å) and Tyr172 (3.1 Å) (Fig. 1d). The N7 atom interacts with a water (wat1 in Fig. 1d; 2.7 Å), which participates in a hydrogen bonding network involving Glu168, Tyr172, and a water (Wat2 in Fig. 1d). In addition to these four hydrogen bonds, the adenine ring forms a T-stacking interaction with the side chain of Phe98, which is fixed by π–π stacking with that of Phe149 (Fig. 1d). The two hydroxyl groups of the ribose moiety of AdoMet interact with the side chain of Glu120 (2.6 and 2.7 Å; Fig. 1d). The methionine moiety of AdoMet forms three hydrogen bonds (Fig. 1d): its amino group hydrogen bonds with the side chain of Asp165 (2.9 Å), and its carboxyl group hydrogen bonds with the main-chain amide nitrogen atoms of Ala104 (3.1 Å) and Leu105 (2.8 Å; Fig. 1d).
We compared the structure of A. aeolicus TrmI to those of T. thermophilus TrmI in complex with S-adenosyl-l-homocysteine (AdoHcy) (Fig. 1e) and P. abyssi TrmI in complex with AdoMet (Fig. 1f), and examined the conservation of residues involved in AdoMet binding. A. aeolicus, T. thermophilus, and P. abyssi all live in high-temperature environments. The N6 amino group of the adenine moiety is recognized in diverse manners by the various TrmI structures. The side chains of three amino acid residues (Asp148, Lys150, and Tyr172 in A. aeolicus TrmI; Fig. 2) surround the N6 amino group, and the underlined residues are involved in AdoMet binding. In the corresponding three positions, T. thermophilus TrmI has Lys153, Glu155, and Val177, while P. abyssi TrmI has Asp153, Tyr155, and Val176 (Fig. 2). Asp148 and Tyr172 of A. aeolicus TrmI form direct hydrogen bonds with the N6 amino group (Fig. 1d). In T. thermophilus TrmI (Fig. 1e) [19], the side chain of Glu155 and a water molecule form hydrogen bonds with the N6 amino group, and these are apparently equivalent to the two hydrogen bonds formed between this moiety and A. aeolicus TrmI. However, Glu155 of T. thermophilus TrmI is located at a different position than Asp148 of A. aeolicus TrmI in the amino acid alignment (Fig. 2). On the other hand, P. abyssi TrmI forms only one hydrogen bond by Asp153 (Fig. 1f) [20], which is located at the same position as Asp148 of A. aeolicus TrmI (Fig. 2). The distances from the N6 amino group to the three water molecules (Fig. 1f) are 3.8, 3.9, and 5.5 Å, respectively. The N7 atom of AdoMet is bound to TrmI by one water-mediated hydrogen bond, although the side chains involved in its coordination differ (Fig. 1d–f).
Sequence alignment of TrmI proteins. The amino acid sequences of A. aeolicus TrmI (AaTrmI), T. maritima TrmI (TmTrmI), T. thermophilus TrmI (TtTrmI), M. tuberculosis TrmI (MtTrmI), P. abyssi TrmI (PaTrmI), and H. sapiens Trm61 (HsTrm61) were aligned with ClustalX 2.1 [31]. Identical residues are white in a red background. Similar residues are red in blue rectangles. The secondary structures of A. aeolicus TrmI (PDB: 2YVL) and H. sapiens Trm61 (PDB: 2B25) are shown at the top and bottom, respectively. The three amino acid residues with side chains located near the N6 amino group of AdoMet are indicated by orange triangles. The figure was depicted by ESPript [32]
We examined the conservation of these three amino acid residues in the other TrmIs with available structures (Fig. 2). Asp148 of A. aeolicus TrmI is conserved in P. abyssi, T. maritima, M. tuberculosis, and Homo sapiens. Lys153 in T. thermophilus TrmI is an exception. Lys150 of A. aeolicus TrmI is not conserved and does not interact with AdoMet. Glu155 of T. thermophilus TrmI and Tyr155 of P. abyssi TrmI participate in the AdoMet binding in distinct manners. By contrast, Ser175 of H. sapiens Trm61 (PDB ID 2B25) is 4.7 Å away from the N6 amino group, and does not interact with AdoMet. The TrmIs from T. maritima (PDB ID 1O54) and M. tuberculosis (PDB ID 1I9G) have Ser and Ala residues, respectively. Although the only available structure of T. maritima TrmI is the substrate-free form, the Ser residue is located too far away to interact with AdoMet. Tyr172 of A. aeolicus TrmI is conserved in M. maritima TrmI, and is replaced by aliphatic residues in T. thermophilus TrmI, M. tuberculosis TrmI, and P. abyssi TrmI, and by Thr175 in H. sapiens Trm61. The side chain of Thr175 is 5.3 Å away from the N6 amino group (PDB ID 2B25), and its hydroxyl group does not coordinate any water molecules.
Two other differences are the presence of T-stacking by Phe98 in A. aeolicus TrmI (Fig. 1d), and the additional hydrogen bond to the ribose moiety by His130, observed in T. thermophilus TrmI (Fig. 1e). The presence of Phe98 is unique to A. aeolicus TrmI (Fig. 2), whereas the His residue at the corresponding position of His130 in T. thermophilus TrmI is shared by the M. tuberculosis and H. sapiens TrmIs. The binding modes for the other part of AdoMet are quite similar. They involve the hydrogen bond between N1 of the adenine moiety to the main-chain amide nitrogen, the interaction between the two hydroxyl groups of the ribose moiety and the Glu side chain, and the binding to the amino and carboxyl groups of the methionine moiety. For the methionine moiety, the Asp165 that interacts with the amino group is conserved, and the conformations of the main-chain amide groups that interact with the carboxyl group are quite similar.
Summary
We have determined the crystal structure of TrmI from the extremely thermophilic bacterium A. aeolicus, and examined the similarities and differences regarding the recognition of the methyl donor AdoMet by A. aeolicus TrmI and the T. thermophilus and P. abyssi TrmIs. The recognition of the N6 amino group of the adenine moiety was the most diverse feature. Three residues are located where their side chains can approach the N6 amino group. Our comparative structural analyses revealed the different strategies adopted by these thermophilic species to form hydrogen bonds by using acidic and hydrophilic side chains. It is intriguing that the universal substrate AdoMet has become recognized in distinct manners by the TrmIs catalyzing the tRNA m1A58 modification, during the course of evolution.
Abbreviations
- AdoMet:
-
S-Adenosyl-l-methionine
- AdoHcy:
-
S-Adenosyl-l-homocysteine
- m1A:
-
N 1-Methyladenosine
- m1G:
-
N 1-Methylguanosine
- m1I:
-
N 1-Methylinosine
- m3C:
-
N 3-Methylcytidine
- m3Ψ:
-
N 3-Methylpseudouridine
- m3U:
-
N 3-Methyluridine
- IPTG:
-
Isopropyl-1-thio-β-d-galactopyranoside
- tRNA:
-
Transfer RNA
- PDB:
-
Protein Data Bank
- RMSD:
-
Root-mean-square-deviation
References
Sprinzl M, Vassilenko KS (2005) Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res 33(Database issue):D39–D40
Gilboa E, Goff S, Shields A, Yoshimura F, Mitra S, Baltimore D (1979) In vitro synthesis of a 9 kbp terminally redundant DNA carrying the infectivity of Moloney murine leukemia virus. Cell 16(4):863–874
Maden BE (1990) The numerous modified nucleotides in eukaryotic ribosomal RNA. Prog Nucleic Acid Res Mol Biol 39:241–303
Burnett BP, McHenry CS (1997) Posttranscriptional modification of retroviral primers is required for late stages of DNA replication. Proc Natl Acad Sci USA 94(14):7210–7215
Renda MJ, Rosenblatt JD, Klimatcheva E, Demeter LM, Bambara RA, Planelles V (2001) Mutation of the methylated tRNA Lys3 residue A58 disrupts reverse transcription and inhibits replication of human immunodeficiency virus type 1. J Virol 75(20):9671–9678
Helm M, Giege R, Florentz C (1999) A Watson-Crick base-pair-disrupting methyl group (m1A9) is sufficient for cloverleaf folding of human mitochondrial tRNALys. Biochemistry 38(40):13338–13346
Hayrapetyan A, Seidu-Larry S, Helm M (2009) Function of Modified Nucleosides in RNA stabilization. In: Grosjean H (ed) DNA and RNA modification enzymes: comparative structure, mechanism, functions, cellular interactions and evolution, chapter 37. LANDES Biosci, USA
Anderson J, Phan L, Cuesta R, Carlson BA, Pak M, Asano K, Bjork GR, Tamame M, Hinnebusch AG (1998) The essential Gcd10p-Gcd14p nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA. Genes Dev 12(23):3650–3662
Kim SH, Suddath FL, Quigley GJ, McPherson A, Sussman JL, Wang AH, Seeman NC, Rich A (1974) Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science 185(4149):435–440
Robertus JD, Ladner JE, Finch JT, Rhodes D, Brown RS, Clark BF, Klug A (1974) Structure of yeast phenylalanine tRNA at 3 Å resolution. Nature 250(467):546–551
Schevitz RW, Podjarny AD, Krishnamachari N, Hughes JJ, Sigler PB, Sussman JL (1979) Crystal structure of a eukaryotic initiator tRNA. Nature 278(5700):188–190
Droogmans L, Roovers M, Bujnicki JM, Tricot C, Hartsch T, Stalon V, Grosjean H (2003) Cloning and characterization of tRNA (m1A58) methyltransferase (TrmI) from Thermus thermophilus HB27, a protein required for cell growth at extreme temperatures. Nucleic Acids Res 31(8):2148–2156
Sierzputowska-Gracz H, Gopal HD, Agris PF (1986) Comparative structural analysis of 1-methyladenosine, 7-methylguanosine, ethenoadenosine and their protonated salts IV: 1H, 13C, and 15N NMR studies at natural isotope abundance. Nucleic Acids Res 14(19):7783–7801
Agris PF, Sierzputowska-Gracz H, Smith C (1986) Transfer RNA contains sites of localized positive charge: carbon NMR studies of [13C]methyl-enriched Escherichia coli and yeast tRNAPhe. Biochemistry 25(18):5126–5131
Anderson J, Droogmans L (2005) Biosynthesis and function of 1-methyladenosine in transfer RNA. In: Grosjean H (ed) Fine-tuning of RNA functions by modification and editing. Springer, Berlin, pp 121–139
Gupta A, Kumar PH, Dineshkumar TK, Varshney U, Subramanya HS (2001) Crystal structure of Rv2118c: an AdoMet-dependent methyltransferase from Mycobacterium tuberculosis H37Rv. J Mol Biol 312(2):381–391
Bujnicki JM (2001) In silico analysis of the tRNA:m1A58 methyltransferase family: homology-based fold prediction and identification of new members from Eubacteria and Archaea. FEBS Lett 507(2):123–127
Roovers M, Wouters J, Bujnicki JM, Tricot C, Stalon V, Grosjean H, Droogmans L (2004) A primordial RNA modification enzyme: the case of tRNA (m1A) methyltransferase. Nucleic Acids Res 32(2):465–476
Barraud P, Golinelli-Pimpaneau B, Atmanene C, Sanglier S, Van Dorsselaer A, Droogmans L, Dardel F, Tisne C (2008) Crystal structure of Thermus thermophilus tRNA m1A58 methyltransferase and biophysical characterization of its interaction with tRNA. J Mol Biol 377(2):535–550
Guelorget A, Roovers M, Guerineau V, Barbey C, Li X, Golinelli-Pimpaneau B (2010) Insights into the hyperthermostability and unusual region-specificity of archaeal Pyrococcus abyssi tRNA m1A57/58 methyltransferase. Nucleic Acids Res 38(18):6206–6218
Guelorget A, Barraud P, Tisne C, Golinelli-Pimpaneau B (2011) Structural comparison of tRNA m1A58 methyltransferases revealed different molecular strategies to maintain their oligomeric architecture under extreme conditions. BMC Struct Biol 11:48
Baker EN (2007) Structural genomics as an approach towards understanding the biology of tuberculosis. J Struct Funct Genomics 8(2–3):57–65
Sugahara M, Asada Y, Shimizu K, Yamamoto H, Lokanath NK, Mizutani H, Bagautdinov B, Matsuura Y, Taketa M, Kageyama Y, Ono N, Morikawa Y, Tanaka Y, Shimada H, Nakamoto T, Sugahara M, Yamamoto M, Kunishima N (2008) High-throughput crystallization-to-structure pipeline at RIKEN SPring-8 Center. J Struct Funct Genomics 9(1–4):21–28
Yokoyama S, Kigawa T, Shirouzu M, Miyano M, Kuramitsu S (2008) RIKEN structural genomics/proteomics initiative. Tanpakushitsu Kakusan Koso 53(5):632–637
Terwilliger TC (2011) The success of structural genomics. J Struct Funct Genomics 12(2):43–44
Otwinowski Z, Minor W (1997) Processing of X-ray diffraction data collected in oscillation mode. Academic Press, New York
Collaborative Computational Project Number 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr D50:760–763
Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D60:2126–2132
Murshudov GN, Vagin AA, Dodson EJ (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D53:240–255
Schuck P (2000) Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys J 78(3):1606–1619
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics (Oxford, England) 23(21):2947–2948
Gouet P, Courcelle E, Stuart DI, Metoz F (1999) ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics (Oxford, England) 15(4):305–308
Acknowledgments
The authors thank the staff at beamline BL41XU of SPring-8. We also thank Tomoko Nakayama, Mihoko Iizuka, Shingo Saito, Taichi Mishima, Kaori Yamanaka, Kojiro Ake, Takako Imada, Kazuko Maekawa, Chie Hori-Takemoto, Tomomi Kamo-Uchikubo, Ryogo Akasaka, Chizu Kuroishi, and Takaho Terada for clerical assistance, performing structural genomics/proteomics projects, facility maintenance, and experimental assistance. This research was supported by a grant from the Daiichi-Sankyo Foundation of Life Science (12-039 to Y.B.), Grants-in-Aid for Scientific Research in Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (to S.Y.), and by the RIKEN Structural Genomics/Proteomics Initiative (RSGI) in the National Project on Protein Structural and Functional Analyses, MEXT of Japan (to S.Y.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kuratani, M., Yanagisawa, T., Ishii, R. et al. Crystal structure of tRNA m1A58 methyltransferase TrmI from Aquifex aeolicus in complex with S-adenosyl-l-methionine. J Struct Funct Genomics 15, 173–180 (2014). https://doi.org/10.1007/s10969-014-9183-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10969-014-9183-0