Abstract
Adsorptive separation of Gd3+ ions was evaluated using DOWEX-50×8 ion exchange resin. It has shown a maximum adsorption capacity of 228.50 mg/g at optimum pH 5 and room temperature. Separation of Gd isotopes was investigated using packed bed columns of the resin in displacement chromatographic techniques where 1,2-cyclohexanedinitrilotetraacetic acid was used as displacer in the mobile phase. Isotopic analysis data by using MC‒ICP‒MS confirmed that the front end of the Gd adsorption band exhibited enrichment of the heavier isotope (160Gd), while the lighter isotopes (157Gd and 155Gd) were concentrated at the rear end of the stationary phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natural Gd consists of seven stable isotopes: 152, 154, 155, 157, 158, and 160. The abundance ratios of the seven isotopes are 0.20, 2.18, 14.80, 20.47, 15.65, 24.84 and 21.86%, respectively [1]. Its exceptional ability to absorb thermal neutrons makes it a preferred choice as a burnable poison in nuclear reactors. This is due to the high neutron absorption cross sections of the 157Gd (2.54 × 106 barn) and 155Gd (0.609 × 106 barn) isotopes. Although natural Gd is presently used as a burnable poison in nuclear reactors, enriched 155Gd or 157Gd will be increasingly used as a viable neutron poison [2]. In addition, the 152Gd isotope is used in producing radioactive 153Gd isotopes. 153Gd is used for research on osteoporosis and bone thickness estimations. Therefore, a process is needed for the enrichment of Gd isotopes. The standard methods for producing isotopes are electromagnetic enrichment [3,4,5,6,7] (calutron), distillation [8,9,10], and centrifuge enrichment [11, 12]. There are also many other methods, such as plasma separation, laser enrichment, and photochemical enrichment. However, every method has its own limitations in terms of cost and scale of production.
On the other hand, chemical exchange methods can be used for isotope separations. These processes are usually associated with smaller separation coefficient values and thus require more time to obtain enriched products than other physical processes; however, these chemical exchange reactions are naturally feasible equilibrium routes. Therefore, these methods are regarded as energy-efficient processes. Displacement chromatography employing ion exchange, which relies on the establishment of equilibrium between isotopic species across mobile and stationary phases, is a highly effective technique for isotopic separation. These methods have been successfully applied for the isotopic separation of different elements, such as Li [13], N [14], Ca [15], Zn [16,17,18,19], Ce [20], Cu [21, 22], Eu [23, 24], V [25], Nd [26, 27] and Gd [28,29,30]. We previously carried out isotope separation of Zn and Gd using chemical exchange methods along with computational methods [31,32,33,34,35,36,37]. However, to date, CDTA has not been utilized for displacement chromatography of gadolinium isotopes with strong cation exchange (DOWEX-50×8) resin. In the current study, we aimed to explore the use of CDTA as a displacement agent to separate Gd isotopes via DOWEX-50×8 resin. The following details are reported in the present paper.
-
In batch-mode equilibrium studies, the effects of the initial concentration of Gd3+ ions and solution by DOWEX-50×8 resin pH by were reported. The adsorption data were fitted with different adsorption isotherm models.
-
In kinetic studies, the effect of shaking time on the adsorption of Gd3+ ions from aqueous solution by resin at optimized pH and room temperaturewas reported. The data were fitted with kinetic models.
-
In thermodynamics studies, the dependence of adsorption of Gd3+ ions from aqueous solution by the adsorbent resin on the temperature at optimized pH was reported. Thermodynamic parameters were calculated from the van’t Hoff plot and Gibbs- Helmholtz equation.
-
Furthermore, in the chromatographic experiments, the adsorption band of Gd was displaced using CDTA in the column loaded with DOWEX-50×8 resin to study the isotope separation of gadolinium.
Experimental
Chemicals
In this study, cation-exchange resin, DOWEX-50×8 mesh size 20–50 was utilized. Gd(NO3)3 solutions were prepared using Gd2O3 (99.999%), sourced from Otto Chemical Industries. The other reagents were of analytical grade and used without additional purification in the present study.
Elemental analysis
For Gd concentration and isotopic analysis, samples were prepared using AR grade ~ 15.0 M HNO3, > 18 MΩ-cm H2O and ~ 30% hydrogen peroxide. The sample preparation was carried out in a manner similar to our earlier work [38]. Sample aliquots were diluted with 2% HNO3 for Gd elemental analysis. The Gdcontent was measured using an inductively coupled plasma optical emission spectrometer (ICP‒OES) model HORIBA ULTIMA.
Isotopic analysis
For Gd isotope analysis, the sample aliquots were diluted with 2% HNO3 to achieve concentrations ranging from 50 to 100 μg/L. Gd isotope measurements were conducted using a Thermo Fisher Scientific Neptune Plus multicollector inductively coupled plasma mass spectrometer (MC‒ICP‒MS) employing the standard sample introduction system at the Radiogenic Isotope Facility, Department of Geology and Geophysics, Indian Institute of Technology Kharagpur, India. Simultaneous detection of masses 154Gd, 155Gd, 156Gd, 157Gd, 158Gd, and 160Gd was achieved using Faraday cups. Instrumental mass bias and drift were corrected using the standard-sample-standard bracketing (SSB) technique. [38]. The mass bias factors were calculated assuming 156Gd/160Gd = 0.9400 and using the exponential law. The reproducibility of replicate measurements of the Fluka ICPMS solutions changed in the range of 0.110/00, 0.070/00, 0.020/00, and 0.070/00 for the 155Gd/158Gd, 156Gd/158Gd, 157Gd/158Gd and 160Gd/158Gd ratios, respectively.
Batch adsorption studies of Gd3+ by DOWEX-50×8
Batch adsorption experiments were conducted using DOWEX-50×8 to study Gd ion adsorption. Parameters like pH, contact time, Gd3+ ion concentration, and temperature were varied systematically. A 10000 ppm Gd3+ stock solution was prepared from Gd(NO3)3 in deionized water. A 200 mg of DOWEX-50×8 resin was mixed with Gd ion solution of desired concentration in a screw capped glass bottle at room temperature and the mixture was shaken for 60 min in an orbital incubator shaker (make: IKA, model: KS 4000). After settling down the resin phase on the bottom of the bottle, supernatant aliquot analyzed for Gd concentration using Inductively CoupledPlasma Optical Emission Spectroscopy ICP-OES (make: Horiba Scientific, model: JobinYvonUltima 2). pH studies were done with 5000 ppm Gd concentration, adjusting pH with HNO3 and NaOH solutions. Equilibrium studies covered Gd ion concentrations from 200 to 10,000 ppm. Kinetic studies were conducted at optimum pH and room temperature. Adsorption was tested at temperatures from 30 to 60 °C with constant adsorbent dosage. Adsorption equilibrium data were used for isotherm modeling, and kinetics were analyzed for the relationship between contact time and adsorption data. Thermodynamic properties were determined from temperature-dependent separation results. Adsorption capacity (Qe, mg/g) was calculated using Eq. 1.
Here, Co and Cf denote the initial and final equilibrium concentrations (mg/L) of Gd ions, respectively. V represents the volume of the solution (mL), and m indicates the weight of the adsorbent. Experiments were performed by duplicate and average values were considered for the calculation. The relative standard deviation of the repeat analysis was within 5%.
Chromatographic separation studies of Gd3+ ions by DOWEX-50×8 resin
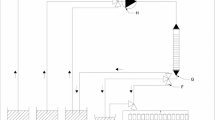
Gadolinium isotope separation by displacement chromatography was carried out in a system for which a schematic of the experimental setup is displayed in Fig. 1. Three glass columns with 10 mm internal diameters (i.d.) and 1000 mm long were connected in series with PTFE tubes (1 mm i.d.). Columns were used repeatedly in a rotational manner (merry-go-round) to achieve the desired migration lengths.
Theoretical models
Isotherm models
Adsorption isotherms can provide useful information such as the adsorption ability of the adsorbent and the type of adsorption [37, 39]. The adsorption equilibrium data were fitted with established two- and three-parameterisotherm models, including the Langmuir [40] (Eq. 2), Freundlich [41] (Eq. 3), Temkin [42] (Eq. 4), Dubinin‒Radushkevich [43, 44] (Eq. 5) and Toth [45] (Eq. 6) models.
In the Langmuir model, qm (mg/g) shows adsorption capacity, and b (L/mg) denotes binding site affinity. The Freundlich model uses Kf and n to determine adsorption capacity and intensity. In the Temkin model, KTe (L/g) and b relate to the constant and heat of adsorption. R is the gas constant, and T is temperature. The Dubinin‒Radushkevich model uses E for adsorption energy. The Toth model uses KTo and n, where n = 1 simplifies to the Langmuir model, otherwise indicating heterogeneous adsorption.
Kinetics models
The adsorption kinetics are investigated, including pseudo-first-order and pseudo-second-order models. The pseudo-first and second-order equations can be expressed as follows:
where qt and qe are the quantity of Gd3+ions adsorbed at time t and equilibrium, k1 (L/min) and k2 (g/mg min) are the first and second order rate constants of adsorption.
Results and discussion
Experimental results
Effect of pH
The pH of the initial solution containing metal ions significantly influences the adsorption process. Figure 2 illustrates the impact of pH on the adsorption of Gd3+ ions (5000 ppm). The adsorption of Gd3+ ion increases notably from pH 1.0 (22.94 mg/g) to 3.0 (190.94 mg/g) and remains constant up to pH 7.0. At lower pH values, thedecrease inadsorption is due to competition between H + and Gd3+ ions. With increase in the pH, the concentration of H+ ions decreases, increasing the availability of Gd3+ ions and subsequently increasing the adsorption capacity. A pH of 5 was identified as optimal for subsequent adsorption investigations.
Effect of initial Gd3+ ion concentration and modeling of the adsorption isotherm
Figure 3 illustrates the calculated adsorption behavior of DOWEX-50×8 relative to the equilibrium concentration of Gd3+ ions. The plot indicates a proportional increase in sorption with the initial concentration of the adsorbate. These findings directly indicate that the uptake capacity of the DOWEX-50×8 resin increases with increasing Ce, leading to more Gd3+ ions being accessible for binding to the adsorbent surface.
Adsorption isotherm models offer valuable insights into the interactions between adsorbates and adsorbents, aiding in the design of effective adsorption systems. The computed parameters for each model, along with the correlation coefficient (R2), are presented. Table 1 presents the fitted models. As per the Langmuir model, the maximum adsorption capacity (qm) of the DOWEX-50×8 resin was determined to be 385.48 mg/g, considerably surpassing the experimental capacity (qm, exp.) of 229.39 mg/g. The Toth isotherm model exhibited the closest fit to the experimental data, as evidenced by its highest R2 value of 0.9914. The maximum adsorption capacity (qm) of DOWEX-50×8 determined by the Toth model was 228.50 mg/g, which closely resembled the experimentally observed value. Notably, Gd3+ adsorption on DOWEX-50×8 manifests as a heterogeneous adsorption process, with an n value deviating from unity.
Kinetic studies
Figure 4 displays the variation in adsorption capacity over time during the adsorption of Gd ions by DOWEX-50×8. The removal of Gd3+ ions by the resin increased with time, peaking at approximately 15 min before stabilizing. Initial adsorption proceeded rapidly until 15 min, with equilibrium achieved by 60 min. Consequently, a shaking duration of 60 min was adopted for all experiments to ensure equilibrium attainment. These data were fitted using kinetic models. The fitted graphs are displayed in Fig. 4, with the calculated parameters presented in Table 2. Both the pseudo-first and second-order models are suitable for describing Gd adsorption on DOWEX-50×8 resin, as indicated by the highest R2 value (0.9267) and maximum adsorption capacity. Moreover, the best fit to pseudo-second-order kinetics indicates that the sorption process is likely chemisorption. This indicates that the rate-limiting step is ion exchange through chemical sorption, involving which the surface-site density of the DOWEX-50×8 resin can be instrumental to the sorption affinity of Gd3+ ions [46].
Adsorption thermodynamics
The significant role of temperature in the adsorption process is well acknowledged. The present observations revealed that the Gd ion adsorption in the resin increased with increasing temperature from 300 to 800 °C. Subsequently, thermodynamic parameters for the adsorption of Gd3+ ions onto the resin were derived from these findings. The enthalpy (ΔH0) and entropy (ΔS0) of adsorption were determined from the van’t Hoff plot (lnKd vs. 1/T), as illustrated in Fig. 5, following Eq. 9.
where Kd is the distribution coefficient of Gd3+ ions by the DOWEX-50×8 resin. The Gibbs free energy (ΔG0) of the adsorption process is given by
The derived values of the thermodynamic parameters are presented in Table 3. These findings demonstrate that the adsorption of Gd3+ ions by the DOWEX-50×8 resin is exothermic, as evidenced by the negative enthalpy of − 5.887 kJ/mol. The observed gibbs free energy values indicates the nature of the adsorption process as spontaneous. Furthermore, a more negative free energy of adsorption suggested the preferred binding of Gd3+ ions by DOWEX-50×8 at temperatures up to 80 °C. The positive entropy change (0.0122 kJ/mol K) coupled with the negative enthalpy change can be attributed to the chemisorption process.
Chromatographic system
The DOWEX-50×8 resin was uniformly packed into the three columns. Prior to usage, the resin underwent pretreatment with 2 M HNO3 solutions, converting it into H + form. Subsequently, a solution containing 0.3 M CuCl2 at pH = 1 was circulated through the columns to transform the resin into Cu2 + form. The equations describing these ion exchange processes can be expressed as follows:
where the underlining represents the resin phase.
A solution of 0.05 M Gd(NO3)3 was passed through the first column on top of the Cu2+ ion band to form a Gd3+ ion adsorption band. Gd3+ ion exchange process can be represented by the equations
After the Gd- band reached a width of 30.0 cm, the passage of the Gd3+ ion solution was closed. Both the Gd3+ ion and Cu2+ ion bands were displaced using 0.05 M (NH4)4CDTA + 0.1 M NH4Cl adjusted to pH 8. The symbol “Z” indicates CDTA. The complexation process of Gd3+ ions with CDTA can be represented as:
The absorption band attributed to Gd3+ ions was distinctly visible and characterized by a pale brown color, which contrasts with the earlier observed blue band indicative of Cu2+ ions. Upon reaching a migration length of 10 m, the Gd3+ ion band was eluted from the final column. The resulting effluent was collected in small fractions and subsequently analyzed for concentration and isotopic ratio.
Changes in the concentration profile and pH of the Gd3+ ion band
As (NH4)4CDTA reaches the leading edge of the Gd3+ ion band, the CDTA ligands undergo transfer to the Gd3+ ion due to the considerably higher stability constant of the Gd3+-CDTA complex in comparison to NH4+. As the Gd-CDTA complex species progress downward through the Gd3+ band within the column, isotopic exchange occurs between the Gd-CDTA complex species and Gd3+ ions present in the resin phase.
Here, X and Y represent lighter and heavier isotopes of gadolinium, respectively.
Subsequently, the Gd-ligand complex advances to the Cu2+ ion band, where CDTA is transferred to Cu2+ ions, facilitating the adsorption of Gd3+ ions onto the resin phase through exchange reactions.
The separation factor, α = 1 + ε, for the Gd isotope pair (for Eq. 17) can be represented as:
The separation coefficients, denoted as ε, were determined utilizing the equation formulated by Spedding et al. [47] and Kakihana and Kanzaki [48]:
where q represents the amount of Gd in the sample volume, Q represents the total capacity of sorbed Gd in the packed column, and rj and ro represent the isotopic ratios of the LGd/HGd jth fraction and the feed, respectively.
The concentration profile of Gd within the effluent fractions following a migration length of 10 m is depicted in Fig. 6, accompanied by the corresponding pH values of the effluent, as illustrated in Fig. 7. The clear demarcations of the band observed in Fig. 6 are complemented by the pH alterations, indicating similar displacements at both the leading and trailing boundaries.
The Gd isotopic fractionation values in the Gd band are given in Fig. 8. The calculated separation coefficient values are presented in Table 4.
From Table 4, it is observed that the lighter isotopes, i.e., 155Gd or 157Gd, are depleted in the front end of the sample fractions, which verifies that the rear end of the Gd3+-band is enriched with lighter isotopes compared to that of natural gadolinium. The separation coefficients, ε × 104, were 0.935, 1.470, 0.382, 2.420, 2.07 and 1.54 for the Gd155/158, Gd156/158, Gd157/158, Gd160/158 Gd155/160, and Gd157/160 Isotope Pairs, respectively. The present calculated ' ε ' values are compared with the earier reported experimental values. The present experimental values are found to be higher compared to the earlier reported values [28, 29, 36] for Gd157/160 isotopic pair with cation-exchange resins (Table 4).
Conclusion
Batch adsorption studies for gadolinium adsorption were carried out by varying the pH, concentration and temperature of the strong cation exchange resin. The optimum pH for the efficient adsorption of Gd3+ ions was found to be 5. The equilibrium time for the maximum adsorption of Gd3+ ions was determined to be 60 min. The adsorption of Gd3+ ions with the DOWEX-50×8 resin is an exothermic process, as revealed by the negative enthalpy of adsorption. A negative Gibbs free energy indicates the spontaneous nature of the adsorption process. From the thermodynamic study, it is concluded that the adsorption efficiency of Gd3+ ions increases with increasing temperature from 300 to 800 °C. The best fit to pseudo-second-order kinetics indicates that the nature of the sorption process is chemisorption. The adsorption of Gd3+ on DOWEX-50×8 occurred heterogeneously. Furthermore, from the displacement chromatography experiment conducted for Gd isotope separation using DOWEX-50×8 resin with the CDTA ligand as a complex-forming agent, the displacement band is enriched with lighter and heavier isotopes in the rear and front ends, respectively. The separation coefficients, ε × 104, were 0.935, 1.470, 0.382, 2.420, 2.07 and 1.54 for the Gd155/158, Gd156/158, Gd157/158, Gd160/158 Gd155/160, and Gd157/160 Isotope Pairs, respectively.
References
Berglund M, Wieser ME (2009) Isotopic compositions of the elements 2009 (IUPAC Technical Report). Pure Appl Chem 83:397–410
Sears VF (1984) Thermal-neutron scattering lengths and cross sections for condensed-matter research. Chalk River Nuclear Laboratories Chalk River (Ontario)
Egle BJ, Hart KJ (2014) Aaron WS. Stable isotope enrichment capabilities at oak ridge national laboratory. J Radioanal Nucl Chem 299:995–999
Tracy JG (1989) Isotope separation program—present and future. Nucl Instrum Methods Phys Res Sect A 282:261–266
Tracy JG, Aaron WS (1993) Stable isotope enrichment-current and future potential. Nucl Instrum Methods Phys Res Sect A 334:45–50
Tracy JG, Bell WA, Veach AM, Caudill HH, Milton HT (1987) Stable isotope enrichment techniques and ORNL separation status. Nucl Instrum Methods Phys Res Sect B 26:7–11
Keim CP (1952) Electromagnetic separation of stable isotopes. Annu Rev Nucl Sci 1:263–292
Dumitrache DC, Inoan I (2014) De Schutter B An analytic model for a isotope separation process by cryogenic distillation. J Process Control 24(5):463–474
McInteer BB (1980) Isotope separation by distillation: design of a carbon-13 plant. Sep Sci Technol 15:491–508
Abdollahi M, Ahmadi SJ (2014) Application of ideal temperature gradient technology to optimize the chemical exchange and distillation process of boron isotopes separation by (CH3) 2O-BF3 complex. Chem Eng Process Process Intensif 76:26–32
Artyukhov AA, Babichev AP, Knyasev IY, Kravets YM, Kurochkin AV, Popov GE, Rudnev AI, Tikhomirov AV (1997) Centrifugal enrichment of cadmium isotopes as the basis for further experiments on physics of wak interactions. Nucl Instrum Methods Phys Res, Sect A 401:281–288
Sosnin LJ, Suvorov IA, Tcheltsov AN, Rudnev AI (1993) Investigation of centrifugal enrichment of stable nickel isotopes. Nucl Instrum Methods Phys Res Sect A 334:41–42
Hagiwara Z (1969) Enrichment of stable isotopes-4. J Nucl Sci Technol 6:508–513
Ahmadi-Motlagh M, Amini Y, Karimi-Sabet J (2022) Experimental study of nitrogen isotope separation by ion-exchange chromatography: effect of process factors. J Radioanal Nucl Chem 331:309–315. https://doi.org/10.1007/s10967-021-08079-y
Umehara S, Nomura M, Fujii Y, Okumura S, Yano T, Kishimoto T (2018) Calcium isotope separation by band chromatography using 18-crown-6-ether resin. J Nucl Sci Technol 55:1473–1480. https://doi.org/10.1080/00223131.2018.1516579
Ding X, Nomura M, Fujii Y (2010) Zinc isotope effects by chromatographic chelating exchange resin. Prog Nucl Energy 52:164–167
Ding X, Suzuki T, Nomura M, Kim HJ, Sgiyama Y, Fujii Y (2007) Zinc isotope separation in acetone by displacement chromatography using benzo-15-crown-5 resin. J Radioanal Nucl Chem 273:79–84
Xue Y, Zu J, Su X, Liu F, Zhan J, He Y, Chen J (2024) A synthesis of novel phenol formaldehyde type dibenzo-18-crown-6 resin for Zn(II) and zinc isotope separation. J Radioanal Nucl Chem 333:519–530. https://doi.org/10.1007/s10967-023-09271-y
Zu J-H, Pan X-H, Su X-D, Liu F, Zhan J, He Y, Chen J-G (2024) Adsorption of Zn(II) from aqueous solution and separation of zinc isotopes by displacement chromatography using chelating adsorbent. J Chromatogr A 1717:464659. https://doi.org/10.1016/j.chroma.2024.464659
Zhang YH, Gunji S, Nomura M, Fujii Y, Oi T (2005) Observation of cerium isotope fractionation in ion-exchange chromatography of Ce(III)-malate complex. J Chromatogr A 1069:133–139
Ismail I, Matin M, Nomura M, Begum S, Fujii Y (2002) Isotope effects of copper in Cu(II) ligand-exchange systems by ion exchange chromatography. J Ion Exch 13:40–45
Abdul Matin MD, Ismail IM, Nomura M, Fujii Y (2002) Isotope effects of copper in Cu(II) malate ligand exchange system studied by using ion exchange displacement chromatography. Sep Sci Technol 37:2129–2142
Ismail I, Nomura M, Fujii Y (1997) Europium isotope effects in ligand exchange system and electron exchange system. In: Proceedings of the 1997 fall meeting of the atomic energy society of Japan
Ismail IM, Nomura M, Fujii Y (1998) Isotope effects of europium in ligand exchange system and electron exchange system using ion-exchange displacement chromatography. J Chromatogr A 808:185–191
Zhang YH, Nomura M, Aida M, Fujii Y (2003) Separation of vanadium isotopes by ion-exchange chromatography. J Chromatogr A 989:175–182
Ismail I (2012) The effect of temperature on the isotope effects of neodymium observed by ion exchange chromatography. Arab J Nucl Sci Appl 45:281–292
Ismail I (2010) Neodymium isotope effects in nd- citrate chromatographic exchange process. Arab J Nucl Sci Appl 43:333–345
Ismail IM, Fukami A, Nomura M, Fujii Y (2000) Anomaly of 155Gd and 157Gd isotope effects in ligand exchange reactions observed by ion exchange chromatography. Anal Chem 72:2841–2845
Chen J, Nomura M, Fujii Y, Kawakami F, Okamoto M (1992) Gadolinium isotope separation by cation exchange chromatography. J Nucl Sci Technol 29:1086–1092
Fujii T, Yamamoto T, Inagawa J, Gunji K, Watanabe K, Nishizawa K (1999) Nuclear size and shape effect in chemical isotope effect of gadolinium using dicyclohexano-18-crown-6. Solvent Extr Ion Exch 17:1219–1229
Arora SK, Boda A, Joshi JM, Singha Deb AK, Govalkar S, Jha M, Ali SM, Shenoy KT, Ghosh SK, Bhatia RK (2014) Enrichment of Gadolinium isotope: column chromatography study. In: Proceedings of the DAE-BRNS biennial symposium on emerging trends in separation science and technology
Boda A, Singha Deb AK, Ali SM, Shenoy KT, Ghosh SK (2014) Conformational effect of dicyclo-hexano-18-crown-6 on isotopic fractionation of zinc: DFT approach. In: American Institute of Physics Conference Series, pp 1065–1067
Singha Deb AK, Ali SM, Shenoy KT, Ghosh SK (2014) Nano cavity induced isotope separation of zinc: density functional theoretical modeling. J Chem Eng Data 59:2472–2484
Sappidi P, Boda A, Ali SM, Singh JK (2019) Adsorption of gadolinium (Gd3+) ions on the dibenzo crown ether (DBCE) and dicyclo hexano crown ether (DCHCE) grafted on the polystyrene surface: insights from all atom molecular dynamics simulations and experiments. J Phys Chem C 123:12276–12285. https://doi.org/10.1021/acs.jpcc.9b01722
Boda A, Singha Deb AK, Ali SM, Shenoy KT, Mohan S (2017) Molecular engineering of functionalized crown ether resins for the isotopic enrichment of gadolinium: from computer to column chromatography. Mol Syst Des Eng 2:640–652. https://doi.org/10.1039/c7me00076f
Boda A, Arora SK, Singha Deb AK, Jha M, Ali SM, Shenoy KT (2017) Molecular modeling guided isotope separation of gadolinium with strong cation exchange resin using displacement chromatography. Sep Sci Technol (Philadelphia) 52:2300–2307. https://doi.org/10.1080/01496395.2016.1260141
Boda A, Sahu P, Singha Deb AK, Ali SM (2022) DFT, MD simulations and experimental analysis of adsorptive complexation and isotope separation of gadolinium ion with macrocyclic crown ether embedded polymeric resin. Sep Purif Technol 289:120709. https://doi.org/10.1016/j.seppur.2022.120709
Singha Deb AK, Sahu P, Boda A, Ali SM, Shenoy KT, Upadhyay D (2020) DFT and MD simulation supplemented experiments for isotopic fractionation of zinc compounds using a macrocyclic crown ether appended polymeric resin. Phys Chem Chem Phys 22:14682–14693. https://doi.org/10.1039/d0cp01660h
Patiha HE, Hidayat Y, Firdaus M (2016) The langmuir isotherm adsorption equation: the monolayer approach. Mater Sci Eng 107:012067. https://doi.org/10.1088/1757-899x/107/1/012067
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38:2221–2295. https://doi.org/10.1021/ja02268a002
Freundlich H (1906) Over the adsorption in solution. J Phys Chem 57:1100–1107
Temkin MI (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta physiochim URSS 12:327–356
Dubinin M (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem Rev 60:235–241
Dubinin MM (1947) The equation of the characteristic curve of activated charcoal. In: Dokl. Akad. Nauk. SSSR, pp 327–329
Toth J (1971) State equation of the solid-gas interface layers. Acta chim hung 69:311–328
Koksal E, Afsin B, Tabak A, Caglar B (2020) Butylamine-resadiye bentonite composite characterization. Spectrosc Lett 53:745–750. https://doi.org/10.1080/00387010.2020.1832530
Spedding FH, Powell TE, Svec HJ (1955) A laboratory method for separating nitrogen isotopes by ion exchange. J Am Chem Soc 77:6125–6132
Kakihana H, Kanzaki T (1969) A simplified and generalized method for analyzing chromatographic isotope separation data. Bull Tokyo Inst Technol 90:77–89
Acknowledgements
We are grateful to Prof. D. Upadhyay, Radiogenic Isotope Facility of the Department of Geology and Geophysics, IIT Kharagpur for isotopic analyses.We are thankful to Dr. Sulekha Mukhopadhyay, Head, Chemical Engineering Division and Shri K.T. Shenoy, Director, Chemical Engineering Group for continuous support and encouragement.
Funding
Open access funding provided by Department of Atomic Energy.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boda, A., Deb, A.K.S., Ali, S.M. et al. Displacement chromatography of gadolinium isotopes with strong cation exchange resin using 1,2-cyclohexanedinitrilotetraacetic acid (CDTA). J Radioanal Nucl Chem (2024). https://doi.org/10.1007/s10967-024-09587-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10967-024-09587-3