Abstract
The present study investigates to incorporate spiked sulfate solution simulate into polymer–cement composite (PCC) based on recycled expanded polystyrene foam. The main aim is to convert the waste stream into leach resistance solid forms able to slow down or even to retard the back release of hazardous radionuclides to the surrounding. Effective parameters e.g. leachant medium, temperature, radioactivity contents, leachant volumes and radionuclides speciation versus the leaching time were studied. The incremental leach rate (Rn, cm/day) and the leach index (Lx) were evaluated for the final waste form after 145 days. The experimental results revealed that the Lx for the all variances can fulfil the waste acceptance criteria for the disposal facility and are above the threshold value of 6. Moreover, leach rate percentages for Cs-134 and Co-60 were not exceeding 2%. The acquired data, based on lab leaching experiments, can recommend the developed PCC under consideration for solidification of radioactive sulphate waste stream safely.
Similar content being viewed by others
Introduction
Nuclear energy has been of notable significance in the issue of sustainable technological development and a greater necessitates for it as a smart source, besides, it is low emission of greenhouse gases. However, the radioactive waste generated is involved in the same topic and should be appropriately handle.
Portland cement is currently being utilized on commercial scale in multiple applications though out the world, including hazard and radioactive waste containment. Incorporation of low- and medium level radwastes into Ordinary Portland Cement (OPC) has been implemented for many years due to the reasonably price easily available materials involved, the besides simple inexpensive processing methods. [1] To improve the properties of a cement-waste containing matrix, particularly its poor resistance to leaching of radionuclides, many organic and inorganic additives have been applied worldwide to obtain inert matrixes have acceptable leaching integrities. [2,3,4,5,6]
Radionuclide release is dependent on the physical and chemical properties and waste packages in addition to, the environmental conditions. Leachability relates to the release of pollutant radionuclides from the waste or solidified waste form, when it comes in contact with solution, into the surrounding environment. The capability of a solid waste form to resist this release is a crucial consideration for disposal sites environment. According to International Atomic Energy Agency (IAEA), it is reported that leach resistance is one of the highest interest properties characterizing the final waste form. [7]
Most hypotheses during disposal of the final waste forms involve the risk that they can be, in the long run, exposed to water intrusion. It is possible, also, that during transport accidents the solidified waste form could come into contact with water. Therefore, capability to retain radionuclides is a crucial asset of the final waste form. The main aim of the leach tests is to evaluate, empirically, the release rates of the radioactive pollutant ingredients into the surrounding biosphere.
Radioactive wastes, regardless of their origin and type, must be solidified before their final storage, or even before their intermediate storage, to eliminate the risk of uncontrolled release of radionuclides pollutants to man biosphere to at least substantially reduce such risk. [8]
Present policy on long-term waste isolation is that the waste package, which includes the waste form and the container, should offer a balance to the natural geological barrier until the waste hazard has been, to a great extent, reduced by radioactive decay. [9]
Leaching can proceed through a multiple mechanisms. Commonly leaching is governed by diffusion; however, other mechanisms can involve e.g. dissolution, chemical reactions and/or combinations of them. All those mechanisms are and issue of the specific surface area of the monolith waste form exposed to the leachant. For instance, ignoring radioactive decay, the semi-infinite solution for mass transport by diffusion from a homogeneous medium having a zero surface contamination for all time (t) > 0 can be calculated according to the relation (1):
where,
Σ an = cumulative activity released, Bq;
Ao = Total initial activity contained in the monolith waste form, Bq;
V = solid specimen volume, cm3;
S = solid specimen geometric surface area, cm2;
De = effective diffusivity, cm/day and.
Σ tn = cumulative leach time, day.
The effective diffusivity is a function for the waste form and radionuclide considered under the designees of the test procedure. [10] Laboratory leach experiments data can then be extrapolated to full scale waste forms by proper application of the V/S ratio.
Radioactive sulfate waste streams are generated in the nuclear facilities, mainly, from the regeneration of exhausted spent ion exchange resins which used as filters. Sulfate waste is considered as one of the most problematic radwaste since its direct incorporation into cement, as a solidifying matrix, can cause corrosion of the final waste form leading to back release of the contained radionuclides.
To overcome the high porosity of cement, a newly polymer- cement composite based on polystyrene foam wastes was developed. That composite is not only cost effective one, since it used the waste foam for formulation, but also, it is environment friendly product through get riding of the accumulation of one of the polymer wastes. The formulation process carried out at room temperature, the obtained polymer-cement composite is mold ability, early setting, acceptable mechanical integrity and low porosity of the final waste form are additional advantages of the PCC under consideration.
In this study sulphate waste simulates was prepared similar to that belongs to wet evaporator bottoms from Boiling Water Reactors (BWRs) and, also from the regeneration of radioactive spent ion exchange resins. The obtained waste simulate was incorporated into cement- polystyrene composite. The used polymer was prepared from chemical recycled polystyrene foam. Factors assumed to affect the leaching behaviour of the reached monolith final waste form, e.g. type of leachant, temperature of leaching test, volume of leachant, radionuclide species studied and the radioactivity content, were studied systematically.
Experimental arrangements
Materials
Sulphate waste simulate (SW)
Simulated sulphate waste streams were used for leachability evaluations. Sodium sulphate waste simulate solution was formulated comparable to the waste slurry generated from BWRs power plants [11] Table 1. The salts were added one after the other in distilled water while stirring. The total concentration of the solution was 225 g/l. The sulphate content was 64.84 g/l added as sodium sulphate (Na2SO4). The obtained solution was evaporated under infrared lamp to complete dryness and up to constant mass. The solid SW was immobilized in the PCC at the ratio 11% by the mass of the dry cement powder used.
Ordinary portland cement (OPC)
The OPC utilized for assembling the cement-polymer composite is an Ordinary Portland Cement (CEMI, N ~ 42.5) fabricated after the Egyptian Standard Specification ES-4759–1/2005 and EN 197–1/2004. The oxides concentrations and compound contents of the dry cement powder are presented in Table 2.
Expanded polystyrene foam waste (PSW)
Expanded polystyrene foam is highly wide spread polymer. It is a rigid thermoplastic polymer with closed cell. In the present work, a recycled PSW waste was utilized as an alternative for the virgin ones. It was collected from the nearby dump site and washed with detergent and water before recycling.
Leaching medium
Plain-, ground-, and seawater were applied as leachant media to evaluate the leach resistance of the polymer-cement composite immobilizing radioactive sulphate waste simulate end products. The concentrations of some ions of interest in the three media are depicted in Table 3.
The PCC—sulphate final waste forms were leached in the three water that simulating, the surface water and assumed to break through the disposal sites.
Radioisotopes used
Simulated waste form monolith formulated for the leachability resistance analyses were spiked with radioactive isotope tracers. Radiocesium (Cs-134, T½ = 2.5 years) and radiocobalt (Co-60, T½ = 5.25 years) which were obtained by irradiating analar grade of cesium chloride (CsCl) and cobalt chloride (CoCl2) in the second Egyptian research reactor.
Salts and solvents
All the salts and solvents applied were of commercial grade and used without further purification otherwise noted.
Methodology
Dissolution of expanded polystyrene foam waste
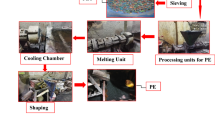
After washing the PSW with detergent, rinsing with water and drying, acetone was added to reduce its volume and convert it to collapse polymer blocks. Those blocks were then dissolved in a small amount of toluene. The resulting resin (PSW-acetone -toluene) compiler at (A: T, 4:2 by volume) can be easily mixed with cement paste because acetone formed major fraction of the resin, which completely miscible with water of the cement paste. The dissolution process was performed at room temperature (30 ± 2 °C). The obtained resin was ready to be mixed with the cement paste. Complete characterization of the obtained resin and its PCC with the cement paste were evaluated in previously published work. [12, 13]
Preparation of the radioactive final waste form
Cement paste was prepared by hydrating dry cement powder with plain water at water: cement ratio 35% by mass. The PCC was obtained when 7% of the resulting (PSW: A: T) resin, calculated relative to the mass of the dry cement powder, was dispersed into the cement paste thoroughly. Dried spiked SW powder was then added to the PCC, while keeping stirring, at the ratio of 11% calculated based on the mass of the dry ordinary Portland cement (OPC) powder. Hand agitation was conducted for more 5 min to keep the PSW resin is entirely dispersed into the mixture and to evacuate the air bubbles that may trapped in the final waste forms. The reached admixture was poured into polyethylene mold and closed tightly with its cover allowing the product to set and hard under its moisture at room temperature (25 ± 2 °C) for 28 days. At the end of the curing period, solid free standing radioactive monolithic blocks were demolded, having dimensions: (3.51 ± 0.05 cm) diameter and (3.17 ± 0.06 cm) height, with no drainable free standing water.
Leaching process
The worst case was selected to evaluate the leach resistance of the cement-composite, under consideration, stabilizing spiked sulphate waste simulate. Hence, each of the acquired solid monoliths was hanged in a plastic container and supported in a perforated grid plastic holder in a way that allowing, more or less, the whole external surface of the specimen for the leachant media's exposure. The leaching test was carried out with the liquid–solid volume ratio of 5: 1, or the ratio of the volume of leachant to exposed surface area of the specimen should not exceed 10 cm. Therefore, the block was hanged in 150 ml of a nominated leachant medium, otherwise denoted. All the leaching experiments were carried out on laboratory scale under batch static conditions (i.e. no agitation, no movement, and the leachant was not replaced by fresh one during the whole test period. Allotment volume of the leachant was periodically withdrawn out of the leaching container, counted for radioactivity content then returned to the leaching jar again, Fig. 1. The two Gamma emitters, i.e. radiocesium and radiocobalt in the leachate were directly measured by γ-ray spectrometer of pure NaI crystal 3 × 3 inch, Genie 2000, Gamma Acquisition & Analysis, Canberra. An aging and curing period up to the 28 days in moist atmosphere before the onset of leaching experiments can reduce the leached radionuclide materials. [14]
Leaching tests were performed at ambient temperature otherwise indicated. The leaching solution was counted for radioactivity daily during the first month, once per week in the following month, then once per month during the following six months and then twice per year as long as is considered necessary.
The leachability of radionuclides out from the final waste form was evaluated based on the leachability Index (Lx). Where, Lx is defined according to Eq. (2).
where De is the effective diffusion coefficient (cm/day).
By substituting the slope into Eq. (3), the effective diffusion coefficient [De] can be obtained: [15]
where the slope was calculated, from the relation of cumulative leach rate [Σan/Ao] versus the square root of the cumulative leach time [√ Σ tn], as a vertical distance divided by the horizontal distance between any two points on the line, which is the rate of change along the regression line
where, Σan is the cumulative amount radioactivity released during leaching periods up to tn (Bq),
Ao is the initial amount of radioactivity in the final waste form (Bq);
V is volume of the solid spiked monolith (cm3);
S is the surface area of solid spiked monolith (cm2) and.
Σ tn is the cumulative leach duration, days.
Various small-scale final waste form specimens were applied to predict long-term releases of radiocesium and/or radiocobalt. The formulation of the different specimens is presented in Table 4.
Results and discussion
The main objectives for the predisposal waste management stages are the fabrication of final waste forms or waste packages suitable for interim storage, transportation and final disposal. The final waste form considered as a compartment in a whole nuclear waste disposal arrangement. Its primary task within this system is to grant the first barrier against hazard radionuclide release.
Leaching experiments were searched at laboratory scales to evaluate the factors that can affect the release of Cs-134 and/or Co-60 from cement polystyrene composite monolith incorporating sulphate waste simulates. Radionuclide release is dependent on the physical and chemical properties of the final waste forms, besides the environmental conditions. [16]
The present study relates to a method to evaluate the radionuclide retention properties of PCC solidified radioactive sulphate wastes simulate. Factors that can affect the release of Cs-134 and/or Co-60, e.g: the types of leachant media, temperature of treatment, volume of leachant, radionuclide speciation, total radioactivity contents, as previously stated, and the data reached were represented and discussed systematically.
Impacts of leachant medium
According to Faiz et al. [17] the kinetic study could be calculated based on the radioactivity (Cs-134 & Co-60) cumulative leach fraction percentage (LF %) according to Eq. (4)
At the end of curing and harding period three specimens of the final PCC monoliths spiked with Cs-134 & Co-60 were leached separately in three leachants, namely, plain-, ground- and sea water for 145 days. The data reached were presented in Figs. 2 and 3.
Based on the data reached, the calculated cumulative leach fraction percentages for the final waste form were 0.35, 0.37 and 0.33% in plain-; ground- and seawater, respectively after 145 days. It should to state that not more than 0.37% of the total radioactivity added was leached out from any final waste forms after 145 days. As the leaching percentages of Cs-134 & Co-60 mixture were extremely less than 20%, therefore, the leaching behaviour of radionuclides from polymer-cement composite waste forms approached that of a semi-infinite medium. [18]
Incremental leach rate (Rn)
The incremental leach rates (Rn, cm/day) for the radionuclides leached in the three leachants were calculated based on the coming relation (5), [17], and the data obtained are illustrated in Fig. 3
The Rn rates were high initially in the three leachants but finally the rates were nearly the same up 145 days. The incremental leach rates for the final waste forms were 1.37E-05, 1.44E-05 and 1.18E-05 cm/day in plain-; ground- and seawater, respectively, Fig. 3. The data demonstrated that the nominated matrix, immobilizing spiked sulphate waste, have low Rn values from the onset of the test which recommended it for solidification/ stabilization of radioactive sulphate waste properly. Similar trend was reported by Zhou et al. [19]
It is clear that the cumulative release fraction in percentage (Σan/Ao, %), (Fig. 2), and incremental leaching rates (Rn), (Fig. 3), of the total radioactivity is low for sea water compared to both plain- and ground- water. As the water: cement ratio is low (i.e. < 0.45) the salt content in seawater attack the surficial of the monolith and brucite salt forms an impermeable layer. [20] Moreover, as seawater has the highest salts content (35 g/l) the other two water. That salts could be deposited inside the PCC microspores led to lower release of radioactivity, Fig. 4. Similar trend was published previously. [5]
It is well known that, the effective mass transfer barrier is depending on the stability and durability of the analogous physical matrix and the area accessible for leaching. It should be notified that, the threshold Leach Index recommended the threshold Leach Index recommended should be greater than 6. [21] The calculated leach indexes for radioelements from the spiked PCC waste monoliths in the sea-, ground- and plain water were nearly 11.6. Those values are greater than that recommended by U.S. Environmental Protection Agency (EPA). [21] This suggested the proposed polymer-cement composite, based on the recycled polystyrene foam, for proper solidification/stabilization of sulphate waste streams, generated from BWRs under the different leachant solutions. The Lx of radionuclides fulfilled the waste acceptance criteria (WAC) of the disposal facility, hence validating their disposal feasibility.
It was reported that, the mechanism that fully controls the leaching process can be determined by the linear regression of the logarithm of Rn values that based on CLF versus the logarithm of time in second. [18]
Figure 5 depictes the coefficients of determination (R2) of the fitting straight curves values for logarithmic incremental leach rates (log Rn, cm/sec.) versus the logarithm of time (Σtn in second) in the three leachants. The all values are above 0.995. This indicates that the diffusion can be the driving leaching mechanism during the radionuclides leaching process. [22]
Impact of temperature on leaching behaviour of the spiked final waste form
The leaching test carried out at cold conditions, i.e. at refrigerator is designed to simulate ocean disposal while that performed at ambient conditions to simulate ground- or plain water in shallow land disposal site.
The chemical integrity of spiked final waste as a function of surrounding temperature was evaluated for monolith leached in groundwater at refrigerator ~ 6 ± 0.2 °C and at room condition ~ 25 ± 2 °C. The results obtained are represented in Fig. 6 and Table 5. It clears from Fig. 6 that the cumulative leach fraction at room temperature is higher than that at ~ 6 °C. This can be attributed to the increase in the mobility of radionuclides incorporated into the final waste forms due to the difference in the surrounding temperature. This conclusion was corroborated by the calculated values for cumulative leach fraction percent, leach index, incremental leach fraction and diffusion coefficient, Table 5.
It is clear that cumulative leach fraction percent, diffusion coefficient and incremental leach rate were lower for the test carried out at ~ 6 °C than that performed at room temperature. It is noted, also, that for the both samples that were kept at room temperature and that set aside at refrigerator have Lx great than 6.
Based on the data presented, it can be stated that the PCC under consideration has the capability to work as inert matrix properly for the sulphate waste streams even at disposal site with low surrounding temperature.
The coefficients of determination (R2) of the fitting straight curve values for logarithmic incremental leach rates, (log Rn, cm/sec.), for the test kept at ~ 6° C and at 25 ± 2 °C and leached in groundwater is above 0.995, Fig. 7. This confirms, again, that the diffusion is mostly the driving leaching mechanism during the radionuclides leaching process even at low temperature.
Effect of the volume of leachant to the exposed surface area of the final waste form
To evaluate the impact of the increasing the volume of leachant, keeping the surface area of spiked PCC at ~ 54 ± 1.3 cm2, on the leachability of radionuclides: three specimens were hanged, separately, in three jars had increasing volumes of ground water as 150, 300 and 450 ml keeping the ratio to the surface area of the solid bocks, usually, less than 10. The results obtained are presented in Fig. 8.
It is obvious that increasing the volumes of leachant accompanied by escalation in leach rate percentages. This can be attributed to that: more water can diffuse into the solid specimens leading to more release of radionuclides. Even so still the driving leaching force is remaining diffusion route, Fig. 9.
Data in Table 6 corroborated the trend in Fig. 9 where increasing the volume of leachant /surface area ratio of the final waste forms accompanied by an escalation in diffusivity from 7.49 E-13 to 4.21 E-12 cm/day as increasing the volume of leachant by three folds. In addition the leach index of the radioactivity decreased from 12.13 to 11.38.
Therefore, the ratio of volume of leachant to the exposed surface area of the final waste monolith should not exceed than 10 cm. Besides, in an accident of breakthrough of water into a repository, it is highly crucial to follow the flow rate of water into the site to avoid the release of hazardous radionuclides out of the solidified waste forms to the surrounding. [18]
Laboratory leach experiments data can be extrapolated to full scale final waste forms by the proper application of the volume/surface area of the solid waste form (V/S) ratio. A two hundreds and ten litres waste form has a V/S ratio of 10 0.8 cm. Applying this concept it can predict.
That foremost of a 210 L polymer-cement composite final waste form incorporating sulphate waste stream under similar conditions could provide cumulative fraction releases nearly 6.75% compare to that obtained at laboratory leaching experiments (V/S = 0.56 cm) at all cumulative leach time values. This can candidate the PCC as an inert matrix for that waste category. However, more work is needed to confirm the reliability of extra-plotting of data obtained over shorter experimental periods.
The behavior of radionuclide species during the leachability of the spiked waste forms
To study the behaviour of radionuclides species during the leachability of the spiked final waste forms, sulphate waste simulate samples were spiked separately with Cs-134 or Co-60 prior to their incorporation into the polymer-cement composite. At the end of curing period, the obtained solid monoliths were leached in groundwater for 145 days and the acquired data are presented in Figs. 10 and 11 for Cs-134 and Co-60 respectively.
Radiocesium is a main component of many waste streams having a detectable radiological and radiotoxicity hazards in addition to its most interested chemical characters that are comparatively simple, being monovalent and very soluble cation.
According to Bayoumi more than 70% of the radiocesium original activity was leached from cement waste form. [23] Similarly, previous published figures for radiocesium leached out of cement specimens were about 90% after 25 days. [24] Those figures are highly comparable to less than 50% Cs-134 released from the polymer-cement composite under consideration, Fig. 10A.
The logarithmic incremental leaching rate acquired for sulphate waste simulate spiked with. Cs-134 and incorporated into the polymer cement matrices is depicted in Fig. 10B. It is clear that the trend of the curve is not straight line. This can be described the leaching mechanism of Cs-134 from the nominated matrices is a combination of wash out, diffusion and dissolution processes. This can be confirmed according to Godbee and compere published work, [25], which denoted that: for most of the final waste forms the leach rate can be describe as combination processes when more than 20% leach rate reached and the leaching behaviour could not be estimate as diffusion of the radionuclide species out semi-finite medium. [26]
In addition to radiocesium, radiocobalt is one of most famed radionuclide in many types of radioactive waste streams. The behaviour of Co-60 in leaching of a spiked PCC waste form in groundwater for 145 days was followed and the reached results were presented in Fig. 11.
On contrary to radiocesium, the total leach fraction percentage of radiocobalt after 145 days was less than 1.6% of the original activity added, Fig. 11A. Similar trends were published. [27,28,29,30,31] The predominate Co-60 leaching deriving force seemed to be generally diffusion as shown in Fig. 11B. However the calculated leaching indices of radiocesium and radiocobalt were 7.91 and 10.25, respectively. Again those values satisfy the Waste Acceptance Criterion (WAC) i.e. < 6. These findings reveal that PCC under consideration can be applied to solidified sulphate wastes generated from BWRs and other peaceful applications of nuclear technologies spiked with radiocesium and radiocobalt appropriately.
Generally, the reduction in the leachability of Cs-134, compared to the published figures, and Co-60 can be due to the occurrence of hydroxide from some heavy metals such as iron and manganese in the cement environment, which can be serving as sorbents and delay the leaching of the two radionuclides. Similar explanation was added after Engelsen et. al. [32]
The significant difference in the leachability of Cs-134 and Co-60 can be explained as follows:
-
(1)
The significant high release of Cs-134 from the PCC monolith can be attributed to the low sorption capability of cement toward radiocesium and its high solubility at the alkaline pH of hydrated cement ranging from 12.5 to 13.6. [33, 34]
-
(2)
Jiang et. al. [35], identified two types of sorbed ions at the calcium silicate hydrate C–S–H surface: the strong inner-sphere and the weak outer-sphere sorbed. It is assumed that radiocesium is strongly sorbed by the outer-sphere surface site where Cs nuclide is loosely bound. [36]
-
(3)
On the other hand the highly significant low leachability of radiocobalt can be attributed to the notably high pH values of the leachates, in the range of 11 and 13 due to the cement hydration products i. e. the presence of soluble Ca(OH)2 and CaCO3 in the cement final waste forms surroundings. Hence it can be proposed that, under this high alkaline conditions, cobalt mostly can be precipitated as hydroxides (Co (OH)2).
-
(4)
In addition, the formation of Co–Al layered double hydroxide (LDH) phases, under the stated cement alkaline condition which can, also, stabilize radiocobalt. Moreover, the metallic ions can be incorporated into the hydrate phases of C–S–H, and mono sulphate (AFm) in the cement grout. [22]
The impact of increasing the incorporated radioactivity on the leached rate
It is obvious from Fig. 12A that there were no significant differences in the total activity leached as function of escalating the radioactivities contents of the spiked sulphate waste simulates incorporated in the PCC and leached in groundwater up to 145 days. Moreover, the incremental leach rates (Rn, cm/day) for the three specimens tested were in the range of -0E5 even by raising the total activity from 1644 Bq/g up to 2791 Bq/g. Also, the leach indexes for the tested activity content namely: 1644, 2255 and 2791 Bq/g were 11.41, 11.82, and 12.13 respectively, Table 7. Those values are satisfying the WAC required for the safe disposal process i.e. ˃ 6.
It should be notified that even by increasing the radioactivity concentrations in the PCC monolith leached in groundwater is, predominantly, the diffusion mode which stills the driving concept describing the leaching process, Fig. 12B.
Conclusion
Based on the data obtained from this study, it was found that the mechanism involved in the leaching of Cs-134 from the polymer cement composite matrix can be surface wash-off, dissolution and diffusion mechanisms, while for Co-60 it can consider as diffusion process. The leachability indexes for the all formulated final waste forms were above the recommended minimum of 6 that allowed their acceptance for safe disposal.
Leachability resistant characteristics of the solidified waste form can be taken as indication for its radiation stability due to the impacts of irradiation from enclosed radioactivity and that nearby in the disposal site.
The application of polymer-cement composite, based on recycled polystyrene foam, as a system for immobilization of sulphate waste originated from Boiling Water Reactors and other peaceful applications in nuclear facilities demonstrate that it is possible to provide not only a higher level of filling with polymer ingredient but also improving the capability of that matrix to stabilize hazard radiocesium waste solutions to acceptable extent. Moreover providing their long term storage stability, during which the water-resistance rise in the course of time.
Based on the leaching characterization and from an environmental point of view, economic reward and cost -effectiveness, it is possible to combine the recycled polystyrene foam to cement paste for a monolith composite matrix suitable for solidification /stabilization of radioactive sulphate waste streams, consequently, can reduce the hazard impacts of the release of radiopollutants to the ecology.
The calculated diffusion coefficients (De) and leachability indexes (Lx) of cesium and cobalt suggested that PCC can be considered as a potentially efficient matrix for both radionuclides immobilization, since the mean leachability index in all cases was above the threshold values and greater than 6.
References
Komljenović M, Tanasijević G, Džunuzović N, Provis JL (2020) J Hazard Mater 388:121765. https://doi.org/10.1016/j.jhazmat.2019.121765
Eskander SB, Tawfik ME (2014) New development in polymer composites research and applications of polymer matrices for solidification stabilization of radioactive wastes. In: Laske S Witschnigg (ed) New Developments in Polymer Composites. Nova Publishers, , New York, pp 33–66
Eskander SB, Bayoumi TA, Saleh HM (2012) J Nucl Mater 420:175–181. https://doi.org/10.1016/j.jnucmat.2011.09.029
Ghattas NK, Eskander SB, Bayoumi TA, Saleh HM (2012) Int J Chem Environ Eng Syst 3:17–25
El-Sayed MI, Eskander SB (2013) Immobilization of organic radioactive wastes, spent liquid waste in cement matrices. LAP Lambert Academic Publishing
Eskander SB, Abdel Aziz SM, El-Didamony H, El-Sayed MI (2011) J Hazard Mater 190:969–979. https://doi.org/10.1016/j.jhazmat.2011.04.036
International Atomic Energy Agency (1985) Treatment of spent ion-exchange resins for storage and disposal, Technical Reports Series No. 254, IAEA, Vienna
Kunze S, Loesch G, Dippel T, Laske D, Huebner W (1986) Method for improving the radionuclide retention properties of solidified radioactive wastes, U. S. Patent Number: 4, 594,186
Merz ER, Dyckerhoff D, Odoj R (1987) Nucl J Can. 1(2):173–178
Colombo P, Neilson RM Jr (1977) Properties of radioactive wastes and waste containers, First topical report, BNL-NUREG-50957. Upton, NewYork, p 11973
Hsu CC, Chiou SL, Quo LW, Kuo ST (1987) Fly ash used in solidification of highly concentrated sulfate chemical waste, In: Proceedings—eighth international ash utilization symposium, Vol 1 Washington, D.C (USA). RN: EPRI-CS--5362-Vol 1
Eskander SB, Tawfik ME (2011) Polym Compos 32:1430
Bayoumi TA, Tawfik ME (2017) Polym Compos 38:637. https://doi.org/10.1002/pc.23622
Rüdolph G, Köster R (1978) Solidification of radioactive waste by inorganic binders, Literature Survey. Kernforschungszentrum Karlsruhe, GMBH, KfK 2535e, ISSN 0303–4003.
Shon JS, Lee HK, Kim GY, Kim TJ, Ahn BG (2022) Materials 15:872. https://doi.org/10.3390/ma15030872
International Atomic Energy Agency. (2013) The behaviours of cementitious materials in long term storage and disposal of radioactive waste, Results of a Coordinated Research Project, IAEA-TECDOC-1701, Vienna, Austria.
Faiz Z, Fakhi S, Bouih A, Outayad R, Benkdad A, Hannache H (2016). Int J Environ Sci Technol. https://doi.org/10.1007/s13762-016-1203-0
Zalina L, Muhamad Samudi Y, Mohd Abd Wahab Y (2016) J Teknologi 78:59–67
Zhou Y, Yun G, Ye Y (2002) Tsinghua Sci Technol 7:636–640
Raimi Lateef Ige. (2010) Effect of Sea water Concentration on Compressive Strength of Concrete. Department of Civil Engineering, College of Engineering, University of Agriculture, Abeokuta
U.S. Environmental Protection Agency (EPA). (1996) Stabilization/ Solidification processes for mixed waste, Center for Remediation Technology and Tools Radiation Protection Division Office of Radiation and Indoor Air, Washington
Yang Z, Ru J, Liu L, Wang X, Zhang Z (2018) RSC Adv 8:27602. https://doi.org/10.1039/c8RA02773K
Bayoumi TA (1997) Study and improvement of the retention capability of some cement barrier on the disposal of low and intermediate-level radioactive wastes, Ph. D. Thesis, Ain Shams Uni. Collage of women, Chemistry Department
Crank J (1976) The Mathematics of diffusion, (Oxford Science Publications) 2nd Edition Uni Press London, 30–31 ISBN-10: 9780198534112
Godbee H, Compere E (1979) Trans Am Nucl Soc 33:791103
Bae K-S, Lee J-O (1997) Environ Eng Res 2:61–72
Eskander SB, Abd El-Aziz SM, El-Sayaad H, Saleh MH (2012) International Scholarly Research Network. ISRN Chemical Engineering Article ID 329676:6. https://doi.org/10.5402/2012/329676
Plecćaš I, Dimovic S (2006) Prog Nucl Energy 48:629–633. https://doi.org/10.1016/j.pnucene.2006.06.012
Pyo JY, Um W, Heo J (2021) J Nucl Eng and Technol 53(2021):2261–2267. https://doi.org/10.1016/j.net.2021.01.005
Zhou Y, Yun G, Yucai YE (2002) Isinghua Sci Technol 7(6):636–640
Gan X, Lin M, Bao L, Zhang Y, Zhang Z (2008) J Nucl Sci and Technol 45:1084–1090. https://doi.org/10.1080/18811248.2008.9711896
Engelsen CJ, Van der Sloot HA, Wibetoe G, Justnes H, Lund W, Stoltenberg-Hansson E (2010) Cem Concr Res 40:1639–1649. https://doi.org/10.1016/j.cemconres.2010.08.001
Soler JM (2003) Appl Geochem 18:1555–1571. https://doi.org/10.1016/S0883-2927(03)00048-9
Ann KY, Kim TH, Kim JH, Kim SH (2010) Constr Build Mater 24:1502–1510. https://doi.org/10.1016/j.conbuildmat.2010.01.022
Jiang J, Wang P, Hou D (2017) J Physical chemistry Chemical Physics 19:27974–27986. https://doi.org/10.1039/c7cp05437h
Arayro J, Dufresne A, Zhou T, Ioannidou K, Ulm JF, Pellenq R, Béland LK (2018) Phys Rev Mater 2:053608. https://doi.org/10.1103/PhysRevMaterials.2.053608
Acknowledgements
The authors express their cordial thanks to the Department of Radioisotope, Egyptian Atomic Energy Authority, for providing facilities to carry out this work. The authors express, also, their deep thanks to the Administration of the National Research Centre, Egypt, for providing funding and all possible facilities to carry out this work. This work is supported by Fund under project number 12010301.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eskander, S.B., Bayoumi, T.A. & Tawfik, M.E. Immobilization of radioactive sulphate waste simulate in polymer–cement composite based on recycled expanded polystyrene foam: evaluation of the final waste form resistance for Cs-134 and Co-60 leachability. J Radioanal Nucl Chem 333, 1851–1863 (2024). https://doi.org/10.1007/s10967-024-09437-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-024-09437-2