Abstract
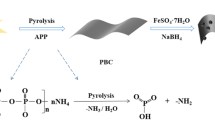
A simple approach for preparing high-efficiency and cost-effective adsorbent to extract uranium in nuclear wastewater is essential for safeguarding the environment. This study introduces an easy procedure to synthesize phosphate-rich biochar (PS) from sawdust by treating it with phosphoric acid before carbonization (700 °C). The sample was characterized by SEM, FT-IR, BET and XPS, indicating that the PS700 exhibited a high concentration of phosphorous groups, high specific surface area and developed mesoporous structure. Subsequently, the effects of pH (optimal at pH 7), adsorption kinetics (fitted by quasi-second-order), and sorption isotherms (modeled by Langmuir) were investigated. The best uranium (VI) adsorption performance of PS700 is 808 mg g−1 at 40 °C and pH 7. In addition, PS700 shows remarkable selectivity and recyclability for uranium (VI). The sorption mechanism can be attributed to the uranium (VI) binding with phosphate groups on the surface of sorbent, which significantly improve its ability for uranium (VI) adsorption. This study displays a great application prospect of PS700 as innovative adsorbent for the efficient extraction of uranium (VI) from nuclear wastewater.











Similar content being viewed by others
Data availability
Data will be made available on request.
References
Zhang X, Liu R, Wang H et al (2023) Fabrication of phosphate-containing mesoporous carbon for fast and efficient uranium (VI) extraction. Colloids Surf Physicochem Eng Asp 662:130994. https://doi.org/10.1016/j.colsurfa.2023.130994
Cheira MF, Kouraim MN, Zidan IH et al (2020) Adsorption of U(VI) from sulfate solution using montmorillonite/polyamide and nano-titanium oxide/polyamide nanocomposites. J Environ Chem Eng 8:104427. https://doi.org/10.1016/j.jece.2020.104427
Wang S, Wang L, Li Z et al (2021) Highly efficient adsorption and immobilization of U(VI) from aqueous solution by alkalized MXene-supported nanoscale zero-valent iron. J Hazard Mater 408:124949. https://doi.org/10.1016/j.jhazmat.2020.124949
Wang K, Wang F, Chen F et al (2019) One-pot preparation of NaA zeolite microspheres for highly selective and continuous removal of Sr(II) from aqueous solution. ACS Sustain Chem Eng 7:2459–2470. https://doi.org/10.1021/acssuschemeng.8b05349
Foster RI, Amphlett JTM, Kim K-W et al (2020) SOHIO process legacy waste treatment: uranium recovery using ion exchange. J Ind Eng Chem 81:144–152. https://doi.org/10.1016/j.jiec.2019.09.001
Hu R, Xiao J, Wang T et al (2020) Engineering of phosphate-functionalized biochars with highly developed surface area and porosity for efficient and selective extraction of uranium. Chem Eng J 379:122388. https://doi.org/10.1016/j.cej.2019.122388
Wei F, Cao C, Huang P, Song W (2015) A new ion exchange adsorption mechanism between carbonate groups and fluoride ions of basic aluminum carbonate nanospheres. RSC Adv 5:13256–13260. https://doi.org/10.1039/C4RA11018H
Nagano T, Mitamura H, Yamashita Y et al (2014) Continuous liquid-liquid extraction of nickel from simulated electroless nickel plating liquid wastes by using a counter current emulsion flow extractor. Solvent Extr Res Dev Jpn 21:111–117
Dang DH, Novotnik B, Wang W et al (2016) Uranium isotope fractionation during adsorption, (co)precipitation and biotic reduction. Environ Sci 50:12695–12704. https://doi.org/10.1021/acs.est.6b01459
Jun B-M (2021) Purification of uranium-contaminated radioactive water by adsorption: a review on adsorbent materials. Sep Purif Technol 278(119675):2–30. https://doi.org/10.1016/j.seppur.2021.119675
Li P, Wang J, Wang Y et al (2019) Photoconversion of U(VI) by TiO2: an efficient strategy for seawater uranium extraction. Chem Eng J 365:231–241. https://doi.org/10.1016/j.cej.2019.02.013
Estes SL, Powell BA (2020) Enthalpy of uranium adsorption onto hematite. Environ Sci Technol 54:15004–15012. https://doi.org/10.1021/acs.est.0c04429
Oyola Y, Janke CJ, Dai S (2016) Synthesis, development, and testing of high-surface-area polymer-based adsorbents for the selective recovery of uranium from seawater. Ind Eng Chem Res 55:4149–4160. https://doi.org/10.1021/acs.iecr.5b03981
Valencia L, Kumar S, Nomena EM et al (2020) In-situ growth of metal oxide nanoparticles on cellulose nanofibrils for dye removal and antimicrobial applications. ACS Appl Nano Mater 3:7172–7181. https://doi.org/10.1021/acsanm.0c01511
Wang H, Liu R, Wang H et al (2021) High effective enrichment of U( vi ) from aqueous solutions on versatile crystalline carbohydrate polymer-functionalized graphene oxide. Dalton Trans 50:14009–14017. https://doi.org/10.1039/D1DT02497C
Ci Z, Yue Y, Xiao J et al (2023) Spectroscopic and modeling investigation of U(VI) removal mechanism on nanoscale zero-valent iron/clay composites. J Colloid Interface Sci 630:395–403. https://doi.org/10.1016/j.jcis.2022.10.008
Zhang Z-H, Lan J-H, Yuan L-Y et al (2020) Rational construction of porous metal-organic frameworks for uranium(VI) extraction: the strong periodic tendency with a metal node. ACS Appl Mater Interfaces 12:14087–14094. https://doi.org/10.1021/acsami.0c02121
Zhong X, Liang W, Lu Z, Hu B (2020) Highly efficient enrichment mechanism of U(VI) and Eu(III) by covalent organic frameworks with intramolecular hydrogen-bonding from solutions. Appl Surf Sci 504:144403. https://doi.org/10.1016/j.apsusc.2019.144403
Gendy EA, Oyekunle DT, Ali J et al (2021) High-performance removal of radionuclides by porous organic frameworks from the aquatic environment: a review. J Environ Radioact 238–239:106710. https://doi.org/10.1016/j.jenvrad.2021.106710
Xue S, Song J, Wang X et al (2020) A systematic comparison of biogas development and related policies between China and Europe and corresponding insights. Renew Sustain Energy Rev 117:109474. https://doi.org/10.1016/j.rser.2019.109474
Thotagamuge R, Kooh MRR, Mahadi AH et al (2021) Copper modified activated bamboo charcoal to enhance adsorption of heavy metals from industrial wastewater. Environ Nanotechnol Monit Manag 16:100562. https://doi.org/10.1016/j.enmm.2021.100562
Miura A (2018) Mechanism of cesium adsorption by carbonized rice hull and beech sawdust. Separations 5:22. https://doi.org/10.3390/separations5020022
Yanovska E, Ryabchenko K, Ianovska M et al (2014) Adsorption of tungsten, molybdenum, vanadium and chromium from aqueous solutions using pine sawdust-polyaniline composites. Nord Pulp Pap Res J 29:425–433. https://doi.org/10.3183/npprj-2014-29-03-p425-433
Xu Z, Xing Y, Ren A et al (2020) Study on adsorption properties of water hyacinth-derived biochar for uranium (VI). J Radioanal Nucl Chem 324:1317–1327. https://doi.org/10.1007/s10967-020-07160-2
Jin J, Li S, Peng X et al (2018) HNO3 modified biochars for uranium (VI) removal from aqueous solution. Bioresour Technol 256:247–253. https://doi.org/10.1016/j.biortech.2018.02.022
Wang S, Guo W, Gao F et al (2018) Lead and uranium sorptive removal from aqueous solution using magnetic and nonmagnetic fast pyrolysis rice husk biochars. RSC Adv 8:13205–13217. https://doi.org/10.1039/C7RA13540H
Peng Y, Zhang Y, Tan Q, Huang H (2021) Bioinspired construction of uranium ion trap with abundant phosphate functional groups. ACS Appl Mater Interfaces 13:27049–27056. https://doi.org/10.1021/acsami.1c04892
Huang K, Hu C, Tan Q et al (2022) Highly efficient removal of cadmium from aqueous solution by ammonium polyphosphate-modified biochar. Chemosphere 305:135471. https://doi.org/10.1016/j.chemosphere.2022.135471
Yang H, Chen P, Chen W et al (2022) Insight into the formation mechanism of N, P co-doped mesoporous biochar from H3PO4 activation and NH3 modification of biomass. Fuel Process Technol 230:107215. https://doi.org/10.1016/j.fuproc.2022.107215
Zhang L, Yao Z, Zhao L et al (2021) Synthesis and characterization of different activated biochar catalysts for removal of biomass pyrolysis tar. Energy 232:120927. https://doi.org/10.1016/j.energy.2021.120927
Da T-X, Ren H-K, He W-K et al (2022) Prediction of uranium adsorption capacity on biochar by machine learning methods. J Environ Chem Eng 10:108449. https://doi.org/10.1016/j.jece.2022.108449
Ahmed W, Núñez-Delgado A, Mehmood S et al (2021) Highly efficient uranium (VI) capture from aqueous solution by means of a hydroxyapatite-biochar nanocomposite: adsorption behavior and mechanism. Environ Res 201:111518. https://doi.org/10.1016/j.envres.2021.111518
Liu R, Wan Q, Yu Y et al (2023) Polyacrylate/phytic acid hydrogel derived phosphate-rich macroporous carbon foam for high-efficiency uranium adsorption. J Water Process Eng 53:103659. https://doi.org/10.1016/j.jwpe.2023.103659
Dai X, Thi Hong Nhung N, Hamza MF et al (2022) Selective adsorption and recovery of scandium from red mud leachate by using phosphoric acid pre-treated pitaya peel biochar. Sep Purif Technol 292:121043. https://doi.org/10.1016/j.seppur.2022.121043
Yue C, Liu R, Wan Q, Wang H, Liu L, Zhang X (2023) Synthesis of novel phosphate-based hypercrosslinked polymers for efficient uranium extraction from radioactive wastewater. J Water Process Eng 53:103582. https://doi.org/10.1016/j.jwpe.2023.103582
Sun Y, Kang Y, Zhong W et al (2020) A simple phosphorylation modification of hydrothermally cross-linked chitosan for selective and efficient removal of U(VI). J Solid State Chem 292:121731. https://doi.org/10.1016/j.jssc.2020.121731
Xu M, Han X, Wang T et al (2018) Conjugated microporous polymers bearing phosphonate ligands as an efficient sorbent for potential uranium extraction from high-level liquid wastes. J Mater Chem A 6:13894–13900. https://doi.org/10.1039/C8TA02875C
Puziy AM, Poddubnaya OI, Gawdzik B, Tascón JMD (2020) Phosphorus-containing carbons: preparation, properties and utilization. Carbon 157:796–846. https://doi.org/10.1016/j.carbon.2019.10.018
Zhang S, Zhao X, Li B et al (2016) “Stereoscopic” 2D super-microporous phosphazene-based covalent organic framework: design, synthesis and selective sorption towards uranium at high acidic condition. J Hazard Mater 314:95–104. https://doi.org/10.1016/j.jhazmat.2016.04.031
Chouyyok W, Warner CL, Mackie KE et al (2016) Nanostructured metal oxide sorbents for the collection and recovery of uranium from seawater. Ind Eng Chem Res 55:4195–4207. https://doi.org/10.1021/acs.iecr.5b03650
Zhang L, Su J, Yang S et al (2016) Extended X-ray absorption fine structure and density functional theory studies on the complexation mechanism of amidoximate ligand to uranyl carbonate. Ind Eng Chem Res 55:4224–4230. https://doi.org/10.1021/acs.iecr.5b03217
Zhong L, He F, Liu Z et al (2022) Adsorption of uranium (VI) ions from aqueous solution by acrylic and diaminomaleonitrile modified cellulose. Colloids Surf Physicochem Eng Asp 641:128565. https://doi.org/10.1016/j.colsurfa.2022.128565
Grabias E, Majdan M (2017) A DFT study of uranyl hydroxyl complexes: structure and stability of trimers and tetramers. J Radioanal Nucl Chem 313:455–465. https://doi.org/10.1007/s10967-017-5305-z
El-Maghrabi HH, Younes AA, Salem AR et al (2019) Magnetically modified hydroxyapatite nanoparticles for the removal of uranium (VI): preparation, characterization and adsorption optimization. J Hazard Mater 378:120703. https://doi.org/10.1016/j.jhazmat.2019.05.096
Bai J, Ma X, Yan H et al (2020) A novel functional porous organic polymer for the removal of uranium from wastewater. Microporous Mesoporous Mater 306:110441. https://doi.org/10.1016/j.micromeso.2020.110441
Tian Y, Liu L, Ma F et al (2021) Synthesis of phosphorylated hyper-cross-linked polymers and their efficient uranium adsorption in water. J Hazard Mater 419:126538. https://doi.org/10.1016/j.jhazmat.2021.126538
Xu M (2018) Conjugated microporous polymers bearing phosphonate ligands as an efficient sorbent for potential uranium extraction from high-level liquid wastes. J Mater Chem A 6:13894–13900. https://doi.org/10.1039/c8ta02875c
Sun Y, Zhang H, Yuan N et al (2021) Phosphorylated biomass-derived porous carbon material for efficient removal of U(VI) in wastewater. J Hazard Mater 413:125282. https://doi.org/10.1016/j.jhazmat.2021.125282
Ma D, Hu S, Li Y, Xu Z (2020) Adsorption of uranium on phosphoric acid-activated peanut shells. Sep Sci Technol 55:1623–1635. https://doi.org/10.1080/01496395.2019.1606016
Zhou Y, Xiao J, Hu R et al (2020) Engineered phosphorous-functionalized biochar with enhanced porosity using phytic acid-assisted ball milling for efficient and selective uptake of aquatic uranium. J Mol Liq 303:112659. https://doi.org/10.1016/j.molliq.2020.112659
Li F, Li D, Li X et al (2016) Microorganism-derived carbon microspheres for uranium removal from aqueous solution. Chem Eng J 284:630–639. https://doi.org/10.1016/j.cej.2015.09.015
Zhang Z, Liu Y, Cao X, Liang P (2013) Sorption study of uranium on carbon spheres hydrothermal synthesized with glucose from aqueous solution. J Radioanal Nucl Chem 295:1775–1782. https://doi.org/10.1007/s10967-012-2247-3
Budnyak TM, Gładysz-Płaska A, Strizhak AV et al (2018) Imidazole-2yl-phosphonic acid derivative grafted onto mesoporous silica surface as a novel highly effective sorbent for uranium(VI) ion extraction. ACS Appl Mater Interfaces 10:6681–6693. https://doi.org/10.1021/acsami.7b17594
Author information
Authors and Affiliations
Contributions
XW: Investigation, Data curation, Writing—Original draft, Writing—Review & Editing. XW: Conceptualization, Writing—Review & Editing. WB: Investigation, Writing—Review & Editing. XL: Editing and Methodology. YT: Formal analysis and Visualization. LL: Resources, Supervision, Writing—Review & Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, X., Wang, X., Bian, W. et al. Phosphate-functionalized mesoporous carbon for efficient extraction of uranium (VI). J Radioanal Nucl Chem 333, 629–639 (2024). https://doi.org/10.1007/s10967-023-09318-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09318-0