Abstract
The aim of this study was to test two potential tritium determination methods (with and without electrolysis enrichment) suitable for low-level measurements. Both methodologies were applied to water samples from the Baltic Sea and ten Polish rivers providing new data on tritium activity concentration. Optimization steps carried out to the standard method lowered minimum detectable activity concentration (MDC) from 3.1 to 1.8 Bq L–1 (at 95% confidence level) enabling to apply it to environmental water samples. However, electrolytic enrichment method of MDC of 0.20 Bq L–1 provided more accurate results for tritium activity concentration in surface waters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tritium (3H), the radioactive isotope of hydrogen is formed naturally through the nuclear reactions induced by cosmic rays in the upper layers of the atmosphere (in the reactions of neutrons with nitrogen or oxygen gases) [1, 2]. Most of the atmospheric tritium is rapidly oxidized to tritiated water HTO and introduced into hydrologic water cycle. Small amounts of tritium are also formed in the lithosphere in the process in which neutrons produced by the spontaneous fission of uranium are captured by 6Li atoms [3]. Anthropogenic tritium is released to the environment by nuclear weapon testing, spent nuclear fuel reprocessing and nuclear reactors. A large quantity of tritium was released into the atmosphere from thermonuclear weapons tests performed in the 1950s and 1960s resulting in the increase of tritium concentration in the precipitation mostly in the northern hemisphere [2,3,4,5]. Since the signing of the convention on the ban on nuclear tests in the atmosphere in 1963, the levels of tritium in the environment from weapons testing have been gradually decreasing, currently reaching values close to the background level [2, 6,7,8]. Nowadays, tritium concentration in the environment can be locally enhanced by releases from nuclear fuel cycle operations during planned and emergency situations. Most of 3H is released into the environment in the form of vapor or liquid, due to technical difficulties in removing tritium from the effluents. That is why 3H is being one of the main target radionuclides in environmental monitoring around facilities such as nuclear power plants and nuclear fuel reprocessing plants [2]. Another application for environmental tritium analyzes is hydrology, since tritium is also a valuable tracer in hydrological studies, for example for groundwater dating or understanding the groundwater cycling [3, 9, 10].
Although, the background radiation levels of tritium are decreasing, tritium measurements in various components of the environment, including river systems and other surface waters, are still the subject of interest of many researchers [7, 9, 11,12,13,14].
Because of the decrease of the’baseline’ environmental radiation levels of tritium, there is a growing demand for the measurement of lower and lower activity concentrations [4, 6].
For many years researchers have been utilizing alkaline electrolysis as the method allowing to perform tritium enrichment of water samples [3, 6, 15, 16], which tends to be a reliable and mature but rather tedious and time consuming method. Tritium enrichment can be also realized in the apparatus using solid polymer electrolyte membrane, which is used as a medium to selectively carry hydrogen ions towards the cathode. The advantage of this method is that alkalization and neutralization process is not necessary. Studies aiming at exploring the performance of other methods like preparation method using sample combustion system [17] or utilizing liquid scintillation counter (LSC) with 100 mL counting vials [18] and comparing the results to electrolytic enrichment method were also conducted recently. Sample combustion method showed promising results. Its main advantage is its rapidness and the fact that oxidation eliminates color quenching effect and reduces the background, but the initial cost of the equipment is high and it demands a gas supply and use of corrosive reagents. On the other hand, the concept of enlarging sample volume to 50 mL by using LSC system with the larger volume of counting vials seems interesting. The authors achieved MDC of 0.18 Bq L−1 after measurement time of 3600 min, which is comparable to detection limits obtained by utilizing electrolytic enrichment, but the long measurement time together with cost and environmental impact of using large volumes of scintillation cocktails are the main disadvantages of this method. Moreover, time needed for dark adaptation of samples needs also to be considerably extended to 1440 min due to a larger volume of scintillation cocktail used.
Radiation Protection Measurements Laboratory (LPD) of the National Centre for Nuclear Research (NCBJ) has been providing environmental radiation monitoring on the premises and in the vicinity of Nuclear Centre at Otwock since 1958, when operation of the first Polish research nuclear reactor began. Recently, Poland has started developing a nuclear programme. Therefore, new regulations based, among others, on European requirements, that must be implemented by the operators of nuclear facilities, have been introduced to Polish legislation (Regulation of the Council of Ministers of August 9, 2022). New regulations are laying down detailed requirements for the environmental monitoring programme and define a number of mandatory measurements of tritium in matrices such as: groundwater, drainage water, surface water, rainwater (some of which are new in comparison to those included in existing monitoring programme). For this reason, and because in the situation of normal operation the expected levels of tritium in such components are low, the study aiming at introducing a sensitive method becomes crucial for the ability to monitor variability in tritium concentrations (for example to recognize the background levels and to detect any unusual changes). Accurate data regarding tritium activity concentrations are also important for the purpose of the exposure assessment of critical groups and population.
The aim of this study was to examine two potential tritium determination methods allowing to obtain a lower minimum detectable activity concentration (MDC) than the standard method routinely used in Radiation Protection Measurements Laboratory of NCBJ. The standard method is based on the LSC measurement of a previously decolorized and distilled sample.
First tested experimental procedure was the modified standard method optimized for measurement time and cocktail:sample ratio. Second tested method was based on electrolytic enrichment. The measurements were carried out using the liquid scintillation technique. Calibration measurements were performed using certified standard tritium solutions and on the basis of obtained results counting efficiency for both methods and basic parameters: enrichment factor Z, retention factor K and separation factor β describing electrolysis process were determined. Accuracy of the electrolytic enrichment method and standard method was verified by participation in national and international intercomparisons. Both established procedures were employed to test 16 different water samples of coastal seawater and freshwater samples taken from various regions of Poland. Samples were collected from well examined waterbodies as well as from other not explored locations providing new and current data of tritium activity concentration in surface waters in Poland. Obtained results were discussed and compared to available data.
Experimental
Materials and methods
Samples
Two types of water samples (seawater and river water) were collected in this study. Coastal seawater sampling points were located on the open sea in Ostrowo and in the Gdańsk Bay on the headland of Rewa. For comparison one sample taken from Adriatic Sea (of higher salinity) was also tested. River water samples were collected in 14 different sampling locations. Three of the chosen sampling points were located in the vicinity of Nuclear Centre operating the only nuclear research reactor in Poland and are included in the Environmental Monitoring Programme of the NCBJ Nuclear Centre at Otwock. Water samples collected from these points are routinely tested in NCBJ’s LPD but not for the tritium activity concentration (such measurements are carried out, but by a laboratory independent of the reactor operator and commissioned by the National Atomic Energy Agency, PAA). River water samples were also collected downstream and upstream the two biggest Polish rivers that is Vistula River and Oder River. Other samples were collected from Narew River which is a right tributary of the Vistula River and its tributary Bug River. Samples from five smaller rivers in the north-eastern Poland were also taken.
Reagents and equipment
All measurements were performed using liquid scintillation counter Tri – Carb 3180 TR/SL (PerkinElmer, USA) equipped with bismuth germanate (BGO) detector guard and a cooling system.
Samples were prepared in the standard 20 mL polyethylene vials (PerkinElmer, USA) and using Ultima Gold AB and Ultima Gold LLT scintillation cocktails (PerkinElmer, USA). All used reagents (sodium hydroxide, sodium thiosulfate, sodium carbonate) were of analytical grade. Certified standard solution of 3H of activity concentration of 750 ± 15 Bq g–1 at the reference date 15 February 2016 (Certificate No BW/Z-63/04p1/16), purchased from NCBJ Radioisotope Centre POLATOM was used to prepare all test samples.
For the preparation of blank samples distilled deep well water from the water intake near the institute of limited contact with the atmosphere and of proven tritium content below 0.1 Bq L−1 was used. The tritium activity concentration in the blank water (often referred to as ‘dead’ water) was determined by electrolytic enrichment of the sample which volume was enlarged to 600 mL.
Standard method for tritium determination in water samples
The analytical procedure for the standard method consisted of measuring the 50 mL test portion of the sample, decolorization with activated carbon, filtration and distillation after the addition of sodium thiosulfate and sodium carbonate. These reagents were added to provide conditions that allow to separate quenching and interfering components such as volatile iodine or carbon dioxide [19]. Afterwards, an aliquot of 5 mL of the obtained distillate was mixed with 15 mL of Ultima Gold AB scintillation cocktail. Before the LSC measurement samples were dark adapted overnight to prevent photoluminescence effects. The samples were counted for 770 min.
To estimate overall efficiency the same process was repeated for a diluted certified standard solution of a known activity concentration. Blank water was used to prepare the dilutions. Overall efficiency was determined according to the following equation:
where Ns—count rate of distilled standard solution [s–1], B—background count rate [s–1], As—activity of standard sample [Bq].
Minimum detectable activity concentration for the standard method was calculated according to the classical approach based on principles of hypothesis testing presented in Procedures Manual of the Environmental Measurements Laboratory [20] or by Passo and Cook [21] assuming equal background and sample measurement times and that background counts collected are above 70.
To derive the formula to express the minimum detectable activity concentration according to this approach characteristic limits: detection limit and critical level (or decision level) must be defined and several assumptions must be made as described in details in the abovementioned references and other publications [22, 23]. Detection limit Ld can be described as the smallest net signal for which there is a confidence at a specified level that the activity is present.
The resulting formula which describes Ld is an approximation based on assumptions that:
-
the probabilities of making error of first kind α (concluding falsely that the activity is present when it is not) and of second kind β (failing to conclude that the activity is present) are equal and set as 5% as well as the abscissas of the standardized normal distribution for the corresponding probabilities of errors are equal and \(k_{\alpha } = k_{\beta } = k \cong 1.645\);
-
gross sample counts and background counts are close and the standard deviation of net measurement result \(\sigma_{0}\) can be estimated as \(\sigma_{b} \sqrt 2\).
Then the detection limit Ld and can be expressed as:
where σb—standard deviation of background.
The formula for MDC calculation can be obtained by dividing the detection limit Ld by a series of factors such as for example: efficiency, aliquot size, chemical yield, time, unit conversion factors etc.
Such approach was adopted when deriving the formula used in this study for minimum detectable activity concentration calculation, which is expressed as follows:
where B—background count rate [s−1], tb—background measurement time [s], E—overall efficiency, V—volume of the analyzed sample [L]. Factor 4.66 equals to \(2k\sqrt 2\), where \(k \cong 1.645\) is the coverage factor (the abscissa of the standardized normal distribution assuming equal probabilities of errors of the first and second kind of 0.05).
Tritium activity concentration was determined as follows:
where N—sample count rate [s–1], B—background count rate [s–1], E—overall efficiency, V—volume of the analyzed sample [L], f—decay correction factor (\(f = exp\left( {\frac{{{-}0,693 t}}{{T_{1/2} }}} \right)\); t—time elapsed between sampling and measurement [days], T1/2—tritium half-life [days]).
Electrolytic enrichment method
During electrolysis process, due to isotopic effect that affects the rates of chemical reactions at the electrodes, light water molecules are decomposed to form H2 and O2 gases more effectively than HTO molecules. As a result tritium concentration in the remaining solution increases.
The electrolytic enrichment process was carried out in an assembly composed of glass cylindrical cells with a flat section at the bottom, where electrode plates were placed. Cells were equipped with glass head with two outputs: side one for electric wires and upper for the tubing discharging generated gases. The electrodes were a stainless steel cathode and nickel anode plates with dimensions of 120 × 28 mm placed in parallel at the distance of 2.5 mm and separated with the Teflon stoppers. The system was equipped with laboratory power supplies and a cooling unit with thermoregulation.
The enrichment process of the samples realized during this study was divided into three electrolysis runs. In each run there were 5–6 tested samples, one blank sample and one sample spiked with certified standard solution of a known activity of tritium. Prior to electrolysis all samples were distilled to remove impurities that might affect the process or could lead to accumulation of the deposits on the electrodes and cell walls.
The maximum volume of the electrolysis cell was approximately 230 mL. With the assumed initial sample volume of 300 mL, the filling was carried out in two stages. First 200 mL of sample and 10 mL of electrolyte solution (20% NaOH) were added into the cell and then, after about 50 h, another 100 mL of the sample was added. Process was initially carried out at a constant current of 5 A and the current was reduced to 3.5 A when the sample reached the volume of 30 mL. Termination of the process took place at the final volume of 15 mL. The whole process was carried out at a temperature range of 0–3°C to reduce 3H loss due to evaporation. After electrolysis the samples were transferred to round-bottom stainless steel flasks and purified from electrolyte by distillation. Then an aliquot of 10 mL of the obtained distillate was transferred to the scintillation vial containing 10 mL of Ultima Gold LLT scintillation cocktail. Measurement time of 400 min was set. In each of the series of measured samples an additional calibration sample was included, which was not subjected to any chemical preparation. It contained the same solution of a known tritium activity concentration as the sample subjected to electrolysis. All blank and calibration samples were prepared in the same manner and measured in the same conditions as analytical samples.
The basic quantities needed to describe the isotopic enrichment process are tritium enrichment factor Z, volume reduction factor R, retention factor K defined as follows:
where Cf, Ci—final and initial tritium concentration, Vf, Vi—final and initial sample volume.
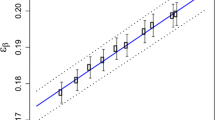
Retention factor K indicates the fraction of tritium that remained in the sample after electrolytic cycle. Separation factor β is the ratio of the concentration of tritium in the aqueous phase and in the gas formed at the cathode [24, 25]:
where: t—number of moles of tritium, p—number of moles of protium.
For a water sample electrolytically enriched the following equation can be derived as presented in the paper of Kaufman and Libby [3]:
which after simple transformation and using Eqs. (5), (6) and (7) gives:
The value of the separation factor β depends on electrode material, type of cells, current density, type of electrolyte and temperature [15, 26]. In the survey of Cameron [15] it was shown that the most common cathode and anode materials are iron and nickel respectively, but comparable results were obtained using other assemblies such as: Ni–Ni, stainless steel-stainless steel, stainless steel-Ni, Pt–Pt.
The abovementioned parameters describing electrolysis process were determined experimentally on the basis of the measurements of certified standard tritium solution which was diluted with dead water to the final activity concentration of 200 ± 3 Bq L−1. Such dilutions were prepared independently for each run. The aliquot of 10 mL of this solution was mixed with scintillation cocktail Ultima Gold LLT and measured directly to determine counting efficiency and to provide data for the calculation of the remaining parameters. Retention factor K was evaluated by the measurement of the samples of standard solution before and after electrolysis and calculated as the ratio of the tritium concentration of enriched to not enriched standard sample.
Tritium activity concentration of electrolytically enriched samples was calculated as follows:
where N—sample count rate [s–1], B—background count rate [s–1], ε—counting efficiency (calculated based on the measurement of undistilled and not enriched standard sample), V—volume of the sample transferred to the counting vial [L], R—volume reduction factor, K—retention factor, f—decay correction factor [defined as in Eq. (4)]. Factor 1.03 is the correction for the addition of 10 mL of electrolyte to the sample resulting in the fact that the tritium concentration is diluted by 3% compared to the initial value.
Minimum detectable activity concentration for electrolyzed samples takes into account the enrichment factor:
where B—background count rate [s−1], tb—background measurement time [s], ε—counting efficiency, V—the sample transferred to the counting vial [L], R—volume reduction factor, K—retention factor. Factor 4.66 is defined as in Eq. (3).
Optimization steps—modification of the standard method
In order to lower the minimum detectable activity concentration achievable by the standard method the following optimization steps were taken.
Firstly, various sample to scintillation cocktail mixing ratios were tested with the aim to maximize the quantity of the sample added, however, not significantly reducing the efficiency, which according to the Eq. (3), would allow to lower the detection limit. The optimal proportion was chosen by determination of the figure of merit (FOM) parameter by counting a series of background and spiked vials with various volumes of sample and liquid scintillation cocktail Ultima Gold AB. Figure of merit was calculated according to the following equation allowing to normalize against the sample volume:
where ε—counting efficiency [%], V—sample volume [mL], B—background count rate [cpm]. Background samples were counted for 200 min. Obtained results are presented in Table 1.
The maximum recommended sample loading volume according to the data provided by the manufacturer [27] is 10 mL. It is not advised to work with the sample loading equal to maximum cocktail holding capacity, while it is dependent on temperature and sample composition. Samples may become cloudy with the change of temperature and it may lead to obtaining falsely low readings. The additional test of sample uptake capacity of Ultima Gold AB was conducted to make sure that the chosen sample volume is correct. Distillate obtained during standard tritium determination procedure was mixed at different ratios of: 7:13; 9:11; 12:8 with the scintillation cocktail in the glass vials for visual inspection. Turbidity of the sample was observed for the ratio 12:8, but for the rest of the samples transparent mixture was obtained, properties of which did not worsen over time, even after several months.
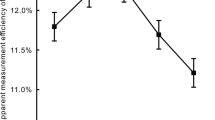
If we assume that for the optimized sample:scintillation cocktail ratio measurements parameters (in particular counting efficiency, sample volume and background count rate) are constant, MDC becomes only the function of measurement time, decreasing as time increases. Moreover, the counting statistics also betters when counting time is prolonged. Therefore, second optimization step taken to lower the MDC of the standard method was the extension of the measurement time above 770 min, which is routinely used for the standard method. This should decrease MDC value and its uncertainty, as well as the uncertainty of tritium activity concentration by decreasing the component of uncertainty of net count rate of the sample which is very important in the case of low-level measurements. Firstly, the dependence of MDC on the measurement time was calculated according to the Eq. (3) on the basis of the results for background counting rate and counting efficiency presented in Table 1 for samples prepared in the following sample:cocktail ratios (5:15; 7:13; 9:11; 10:10). Then longer lasting measurements of background samples were performed to obtain the experimental data, verify and prove the correctness of the predictions. Obtained results are shown in Fig. 1.
Data presented in Table 1 and in Fig. 1 show that the optimal sample to scintillation cocktail ratio was 9:11, because using this proportion the highest FOM value and the lowest MDC value were obtained. The standard deviation of the count rate is dependent on the counting time and decreases as the counting time increases. This is important especially when the numbers of sample counts and backgroung counts are close, because, due to the fact that the standard deviations of sample and background counts are smaller, the uncertainty of their difference (calculated according to the law of uncertainty propagation) is also reduced to an acceptable level. Based on the measurements and calculations carried out, extended measurement time of 1200 min was selected, since assuming that activity concentration of 2.25 Bq L−1 is expected to be found in a water sample (for the conditions given in Table 1 for the chosen 9:11 ratio and the measurement time equal to 1200 min) standard uncertainty of the net count rate that is not exceeding 25% can be reached. Further increase of the measurement time does not significantly decrease neither the MDC value nor the net count uncertainty (for extension from 1200 to 1500 the MDC decreases from 1.81 to 1.62 Bq L−1 but standard uncertainty of the net count rate equals to 22%. Moreover, extending measurement time to the value that significantly exceeds a day does not seem reasonable, because it highly reduces the throughput of the counter. Those parameters were adopted in further analyzes according to modified standard method.
Results and discussion
Determination of parameters describing examined methods
In electrolytic enrichment method performance parameters such as enrichment factor Z, retention factor K, counting efficiency ε are evaluated in each batch.
To characterize the performance of the apparatus and selected method, parameters describing electrolysis process were determined as mean values taking into account ten last electrolysis runs and are presented along with their standard deviations σ in Table 2.
Electrolysis parameters obtained during the surface water study were within the shown range.
Determined enrichment parameters including the enrichment factor Z are in agreement with the results obtained by other researchers for conventional cells. For example in the survey of Cameron [15] separation factor of 12 was indicated as typical and it was summarized that tritium recoveries of about 80% were common for a volume reduction factor of 15, giving tritium enrichment of 12–15 times. In the paper of Plastino et al. [28] for mild steel-stainless steel system determined enrichment factor for the volume reduction from 330 to 25 mL was in the range of 11.0–12.2 and it was raised to the value of about 22, when the sample amount was increased to 660 mL in a two-stage process. From the Ni–Fe conventional cell computed enrichment profile shown in the paper of Soreefan and De Vol [25] it can be read, that for the volume reduction factor of about 21 as was achieved in this work, enrichment factor value of about 18 is obtained. According to Carter et al. [6] elelctrolysis cells are usually designed to achieve enrichment factor from 15 to over 30.
Main parameters describing both methods tested within this study in comparison to standard method are summarized in Table 3. All values were derived on the basis of the measurements performed by liquid scintillation counter Tri-Carb 3180 TR/SL. Calibration measurements for the standard method and modified standard method were performed using certified reference solution of tritium diluted to the activity concentration of 1210 ± 30 Bq L−1. In the case of the standard method, calibration measurements are carried out periodically based on the preparation of series of samples. Efficiency value presented in Table 3 for the standard method is a mean value and represents overall efficiency of the method. Shown efficiency values were presented along with their combined expanded uncertainties. Values of MDC were calculated according to Eq. (3) for standard methods and Eq. (12) for the method of electrolytic enrichment and presented along with their combined expanded uncertainties. Uncertainties of the obtained MDC values were calculated using the law of uncertainty propagation (by calculating the square root of the sum of sensitivity factors, which are the squares of partial derivatives of all individual variables, multiplied by the squares of individual uncertainties of the variables).
Gained MDC values are comparable to the values reported for similar measurement conditions by other researchers. For instance, for electrolytic enrichment method Gomes et al. [7] obtained MDC of 0.4 Bq L–1 using Tri-Carb 3170 TR/SL, 300 min counting time and 8:12 sample:cocktail mixing ratio (Ultima Gold LLT), whereas Grahek et al. [14] reported almost equal to obtained in this study value of 0.19 Bq L–1 (for Tri-Carb 3180 TR/SL, 300 min counting time and UG LLT scintillation cocktail mixed in the proportion of 7 mL of the sample and 13 mL of cocktail). In direct measurements (without enrichment) also values of MDC comparable to obtained in this work of 2.88 Bq L–1 were shown by Grahek et al. [14] for Tri-Carb 3180 TR/SL.
Optimization steps taken consisting in increasing the sample volume and extending the measurement time allowed to lower the MDC value by more than 40% from 3.07 ± 0.10 Bq L−1 to 1.81 ± 0.06 Bq L−1 which is corresponding to about 1.3 Bq L–1of overall MDC decrease. It was calculated that the step of increasing the measuring time from 770 to 1200 min allowed to reduce the MDC from 2.27 ± 0.08 Bq L−1 to 1.81 ± 0.06 Bq L−1 (which gave the decrease of about 0.5 Bq L−1). In comparison, first optimization step of increasing the sample volume allowed to to decrease the MDC by about 0.8 Bq L–1. When comparing these two values, it can be seen that the second optimization step resulted in reduction of MDC by approximately 60% of initial reduction achieved (of 0.8 Bq L−1). Considering the gained MDC results along with their uncertainties, which are equal to 2.27 ± 0.08 Bq L−1 for the measuring time of 770 min and 1.81 ± 0.06 Bq L−1 for the measuring time of 1200 min, they exhibit no overlap. Both uncertainties are also much lower than 0.5 Bq L−1.
The step of increasing the measurement time not only lowered the MDC but also improved the counting statistics. Increasing the measuring time to 1200 min allowed to reduce uncertainty of a single background count rate measurement (expressed as a square root of the quotient of count rate over time) from 2.1 to 1.7%. However, extending the measuring time had the greatest impact on reduction of the uncertainty of net count rate, which in low-level measurements becomes the predominant component in the combined uncertainty. Extended time of 1200 min resulted in obtaining relative standard uncertainty associated with net count rate of 25% instead of 31% for the samples of the activity concentration slightly above the detection limit of about 2.25 Bq L−1. Obtained MDC for the modified method is still far from the value gained for electrolytic enrichment. However, taking into account the possible benefit related to significant reduction of time needed for sample preparation (from 7 days to few hours), makes using a modified method worth considering and testing by performing analyzes of samples of low activity concentrations.
Interlaboratory comparisons
Quality of the results obtained for the analytical methods can be verified by interlaboratory comparisons [7, 29, 30]. Accuracy of the method of electrolytic enrichment and of the standard method for tritium determination was examined by participation in national and international laboratory comparisons on tritium determination in water samples. The first intercomparison was organized at national level by National Atomic Energy Agency (PAA) in Poland. Usually two samples of different activity concentrations are determined by participating laboratories. Main acceptance criterion adopted by the organizers was the \(\left| {Z - score} \right| \le 2\), while \(\left| {\zeta - score} \right| \le 2\) served as the supplementary criterion. The second proficiency test was organized by PROCORAD (Association for the Promotion of Quality Control in Radiotoxicological Analysis) and in principle was dedicated to tritium analysis in urine samples, but each measurement series included also one tritiated water solution. The following evaluation criteria were adopted: the \(\left| {Z - score} \right| \le 2\) as the main performance indicator and \(\left| {E_{n} } \right| \le 1\) and the relative bias within the range − 25% ÷ 50% provided for information purposes only [31].
Results obtained over last years in both intercomparisons are summarized in Table 4. Activity concentrations are shown along with their expanded combined standard uncertainties (k = 2). Symbol n/a was used to indicate that the parameter has not been assessed by the organizers.
All of the reported results met the acceptance criteria of the organizers and were close to the target values. Regardless the tested activity concentration level relative bias did not exceed 10%. Participation in the intercomparisons and satisfactory results obtained repeatedly allowed to prove the accuracy of tested methods.
Calibration measurements and results of interlaboratory comparisons confirmed that the electrolysis system is operational and that the tested methods can be expected to be useful during surface water study.
Surface water measurements
To verify their performance in practice, both investigated analytical procedures were applied to saline and non-saline surface water samples taken from the Baltic Sea, Adriatic Sea and from rivers in different regions of Poland. Seawater and freshwater samples were treated in the same manner, because according to International Standard ISO 9698:2019 (E) [32] this type of method is suitable for many types of water such as surface waters, ground waters, rain waters including marine waters.
The Baltic Sea is a moderately salinized inland sea of salinity of approximately 1% [33] By contrast, the salinity of Mediterranean Sea may exceed that of oceanic water. Before starting tritium analyzes in marine waters, the content of total dissolved solids (TDS) was determined according to the ISO Standard [34] by weighing a filtered sample and drying at 105°C. The following values of TDS content were obtained: 8800 mg L−1 for Baltic Sea in Ostrowo, 9300 mg L−1 for Baltic Sea in Rewa and 44000 mg L−1 for Adriatic Sea in Budva. To check how effectively the distillation process will reduce the dissolved solids content, TDS in the sample of highest salinity (that is in Adriatic Sea water) was also determined after the preparation according to modified tritium determination procedure. The TDS value was reduced to 100 mg L−1 that is 440 times, confirming that sample salinity will not affect analysis.
The results of the determination of tritium activity concentration in 17 water samples prepared and measured using investigated methods are shown in Table 5.
Results of tritium activity concentrations in surface water samples obtained with both tested analytical procedures were comparable when their expanded uncertainties are taken into account.
All of tritium activity concentrations in Polish surface waters collected during this study were found to be at the level above the minimum detectable activity concentration for the electrolytic enrichment method. Only five of the investigated samples showed results just slightly above the minimum detectable activity concentration for the modified method and therefore were characterized by considerable uncertainty. For comparison tritium activity concentration in saline water from Adriatic Sea was also determined and according to both methods it was below the minimum detectable activity concentration, which is consistent with the data of HTO concentrations of waters of Mediterranean Sea (ca. 0.1 Bq L–1) provided by Eyrolle [13].
The lowest activity concentrations were obtained for the small rivers of the north-eastern region of Poland (the lowest value of 0.43 ± 0.16 Bq L–1 was recorded for Sajna River), whereas the highest for biggest Polish rivers Vistula and Oder and in the samples from the Baltic Sea. The highest result according to electrolytic enrichment method was 0.94 ± 0.32 Bq L–1 which was obtained for the sample taken from Oder River in Wrocław According to modified standard method the highest tritium activity concentration of 2.2 ± 1.1 Bq L–1 was observed in Baltic Sea in Gdańsk Bay where also the second highest result of 0.92 ± 0.31 Bq L–1 for enrichment method was obtained. Results at this level of activity are comparable with data provided for southern Baltic Sea water in 2020 where tritium activity concenration of 2.6 ± 1.1 Bq L–1 was determined [35] and are in accordance with the concentrations characteristic for Baltic area which vary from in the range 1.0—4.0 Bq L–1 as it was reported by HELCOM’s Expert Group on Monitoring of Radioactive Substances in the Baltic Sea [36].
Tritiated water concentrations measured in the samples collected from sampling points at the Świder River in the vicinity of NCBJ Nuclear Centre at Otwock were below the values measured for other main Polish rives. Almost equal results were obtained for the samples from both locations that is activity concentration of 0.68 ± 0.24 Bq L–1 for the collection point located upstream from the Nuclear Centre and not influenced by its operation and of 0.67 ± 0.24 Bq L–1 downstream the river. Monitoring data concerning tritium concentration in samples taken from Świder River near Nuclear Centre are also provided by an independent laboratory (Central Laboratory for Radiological Protection in Warsaw) and are reported regularly in Annual reports of the President of the National Atomic Energy Agency (PAA). Results obtained in this study are in agreement with the data published in the PAA’s President Report for 2022 which were given as year averages of 0.6 Bq L–1 upstream from the Centre and 0.9 Bq L–1 downstream.
Determination of tritium concentration in surface waters and precipitation in Poland was the subject of comprehensive study conducted by Radwan et al. [37], when Vistula, Oder, Bug, Narew, Nysa Łużycka and Warta Rivers, six lakes and 10 small rivers of Seashore Region and tap water from Zegrzyński Reservoir were monitored in the period 1994–1999. The paper shows that annual average concentrations in the abovementioned waters (except for Seashore small rivers for which lower concentrations were found with the average of 1.1 Bq L–1) ranged between 1.4 to 1.9 Bq L–1. The concentrations found in precipitation during this period were comparable and ranged from 1.7 to 2.2 Bq L–1. Among river waters, the highest concentration of 2.5 ± 0.6 Bq L–1 was recorded for Warta River in 1995.
Long term studies between 1975 and 2018 of tritium content in monthly precipitation for the Kraków station conducted by Duliński et al. [38] confirmed that in Poland the concentrations of tritium in precipitation are still decreasing reaching nowadays typical for mid-latitude value of about 1 Bq L–1 [39] and revealed the expected seasonal variations of tritium content with maximal values in spring–summer period. These variations reflect seasonal changes in the dynamics of the transport of tritium from stratosphere, where it is accumulated, to the troposphere. Given that in Poland there is no nuclear industry, wet and dry deposition may be considered as main tritium sources.
Therefore, values of tritium activity concentrations in surface waters obtained during this study, which are lower in comparison to the data presented by Radwan et al. [37] obtained until 1999, are in line with this trend of decrease. Moreover, values obtained in this paper are comparable with tritium activity concentrations in surface waters recorded in other neighboring countries in Central Europe. For example, annual average value of tritium concentration in Elbe River basin in Czech Republic also followed the trend of decrease reaching value of 0.9 ± 0.2 Bq L−1 in 2016 as reported by Merešová et al. [39].
In this study the average activity concentration determined for all sampling points at big rivers (Vistula, Oder, Bug and Narew) was 0.85 Bq L–1, whereas for relatively smaller rivers (Szkwa, Łyna, Guber, Sajna and Krutynia) the average of 0.57 Bq L–1 was obtained. These two values are significantly different according to t-Student test. Similar phenomenon was observed in the study of Radwan et al. [37] where conclusion was drawn that this might be connected with relatively smaller drainage area of the small rivers compared to bigger ones leading to smaller tritium amount deposited with precipitation. Results from this work seem to support this thesis.
Conclusions
The optimization of standard method resulted in creation a procedure for which MDC value of 1.8 Bq L–1 was obtainted. The method is applicable to low-level tritium measurements of water samples, but only provided that concentrations above this detection limit are expected. Modified method is considerably faster than electrolytic enrichment method when sample preparation time is taken into account, however it involves the occupation of the apparatus for a long time, which can affect laboratory throughput. The modified method would certainly be more perspective, if it was combined with measurements by ultra-low level liquid scintillation counter.
Determined enrichment parameters of electrolysis system used during the experiment were satisfactory, comparable to literature data for conventional cells and high enough to decrease MDC to the value of 0.20 Bq L–1.
This research presents updated data for tritiated water activity concentrations encountered in freshwater and seawater samples from the territory of Poland. Tritium activity concentrations that were found in surface water samples using both tested methods were in agreement within their expanded uncertainties. According to electrolytic enrichment method obtained tritiated water activity concentrations measured for the investigated surface water samples ranged from 0.43 ± 0.16 to 0.94 ± 0.32 Bq L–1 with the average of 0.85 Bq L–1 for big Polish rivers significantly different from the average of 0.57 Bq L–1 for small rivers in the north-eastern region. Tritium activity concentrations measured in the samples collected from the Świder River upstream and downstream NCBJ Nuclear Centre at Otwock demonstrated (together with the data from PAA’s President Annual Report) that operation of the NCBJ facilities did not contribute to increase in tritium activity concentration in river water in 2022.
Conducted studies have shown that electrolytic enrichment followed by liquid scintillation counting, although more laborious, is the method that the authors have chosen for future tritium analyzes of water samples at activity levels that were found currently in surface waters in Poland. Electrolysis method, provides over five times lower minimum detectable activity concentration and lower uncertainties of the results, therefore it is more accurate and reliable.
References
Lal D, Peters B (1967) Cosmic ray produced radioactivity on the earth. In: Sitte K (ed) Cosmic rays II. Encyclopedia of physics, vol 46. Springer, Berlin, Heidelberg
Momoshima N (2022) Tritium in the environment. Radiat Prot Dosimetry. https://doi.org/10.1093/rpd/ncac002
Kaufman S, Libby WF (1954) The Natural Distribution of Tritium. Phys Rev. https://doi.org/10.1103/PhysRev.93.1337
Theodorson P (1999) A review of low-level tritium systems and sensitivity requirements. Appl Radiat Isotop. https://doi.org/10.1016/S0969-8043(97)10153-1
Kumar B, Han LF, Wassenaar LI, Klaus PM, Kainz GG, Hillengods D, Brummer D, Ahmad M, Belachew DL, Araguas L, Aggarwal P (2016) A compact tritium enrichment unit for large sample volumes with automated re-filling and higher enrichment factor. Appl Radiat Isotop. https://doi.org/10.1016/j.apradiso.2016.07.018
Carter B, Cook GT, Passo CJ (2012) Environmental liqud scintillation analysis. In: L’Annunziata MF (ed) Handbook of Radioactivity Analysis, 3rd edn. Academic Press, Elsevier
Gomes AR, Abrantes J, Libânio A, Madruga MJ, Reis M (2017) Determination of tritium in water using electrolytic enrichment: methodology improvements. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-017-5456-y
Villa M, Manjon G (2004) Low-level measurements of tritium in water. Appl Radiat Isot. https://doi.org/10.1016/j.apradiso.2004.03.027
Schmidt A, Frank G, Stichler W, Duester L, Steinkopff T, Stumpp C (2020) Overview of tritium records from precipitation and surfacewaters in Germany. Hydrol Process. https://doi.org/10.1002/hyp.13691
Michel RL, Aggarwal P, Araguas-Araguas L, Kurttas T, Newman BD, Vitvar T (2015) A simplified approach to analyzing historical and recent tritium data in surface waters. Hydrol Process. https://doi.org/10.1002/hyp.10174
Ducros L, Eyrolle F, Vedova C, Charmasson S, Leblanc M, Mayer A, Babic M, Antonelli C, Mourier D, Giner F (2018) Tritium in river waters from French Mediterranean catchments: Backgound levels and variability. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2017.08.026
Wallova G, Meresova J, Zvachova S, Petranova I, Sykora I (2020) New electrolytic enrichment system for tritium determination in water research institute in Bratislava and IT’S first results of tritium activity in precipitation. J Environ Radioact. https://doi.org/10.1016/j.jenvrad.2020.106177
Eyrolle F, Ducros L, Le Dizès S, Beaugelin-Seiller K, Charmasson S, Boyer P, Cossonnet C (2018) An updated review on tritium in the environment. J Environ Radioact. https://doi.org/10.1016/j.jenvrad.2017.11.001
Grahek Ž, Breznik B, Stojković I, Coha I, Nikolov J, Todorović N (2016) Measurement of tritium in the Sava and Danube Rivers. J Environ Radioact. https://doi.org/10.1016/j.jenvrad.2016.05.014
Cameron JF (1967) A survey of systems for concentration and low background counting of tritium in water. In: Radioactive dating and methods of low-level counting. IAEA-SM-87/25, IAEA, Vienna, p 543
Taylor CB (1981) Present Status and Trends in Electrolytic Enrichment of Low-Level Tritium in Water. In: Methods of Low-Level Counting and Spectrometry. IAEA-SM-252/63, IAEA, Vienna, p 303
Nikolov J, Todorovic N, Jankovic M, Vostinar M, Bikit I, Veskovic M (2013) Different methods for tritium determination in surface water by LSC. Appl Radiat Isot. https://doi.org/10.1016/j.apradiso.2012.09.015
Feng B, Chen B, Zhao C, He L, Tang F, Zhuo W (2020) Application of a liquid scintillation system with 100-ml counting vials for environmental tritium determination: Procedure optimization, performance test, and uncertainty analysis. J Environ Radioact. https://doi.org/10.1016/j.jenvrad.2020.106427
Lehto J, Hou X (2011) Chemistry and analysis of radionuclides. Wiley-VCH, Weinheim
EML (1997) Section 4.5.3 quality control and detection limits. In: Chieco NA (ed) HASL 300 The procedures manual of the environental measurements laboratory Vol. I, 28th edn. U.S. Department of Homeland Security, New York. https://www.wipp.energy.gov/NAMP/EMLLegacy/procman/Start.htm. Accessed 28 Aug 2023
Passo Jr CJ, Cook GT (1994) Handbook of Environmental Liquid Scintillation Spectrometry, A Compilation of Theory and Methods. Publ No. PMC0387. PerkinElmer Life and Analytical Sciences, Boston
Rucker TL (1995) Methodologies for the practical determination and use of method detection limits. J Radioanal Nucl Chem. https://doi.org/10.1007/BF02041739
Hurtgen C, Jerome S, Woods M (2000) Revisiting Currie-how low can we go? Appl Radiat Isot. https://doi.org/10.1016/S0969-8043(00)00171-8
Yoon YY, Lee KY, Ko KS (2010) Development and validation of Ni–Ni electrolytic enrichment method for tritium determination in samples of underground waters of Jeju Island. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-010-0731-1
Soreefan AM, De Vol TA (2009) Determination of tritium enrichment parameters of a commercially available PEM electrolyzer: a comparison with conventional enrichment electrolysis. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-009-0256-7
Kakiuchi M, Tanaka K, Kunihiko K (1991) Natural tritium measurements: determination of the electrolytic enrichment factor of tritium. Int J Radiat Appl Instrum Appl Radiat Isot. https://doi.org/10.1016/0883-2889(91)90178-4
Elder R, Cocktails for Liquid scintillation counting. LSC Application Note, PerkinElmer Inc. https://resources.perkinelmer.com/lab-solutions/resources/docs/app_cocktails-for-liquid-scintillation-counting-011940_01.pdf. Accessed 30 Aug 2023
Plastino W, Chereji I, Cuna S, Kaihola L, De Felice P, Lupsa N, Balas G, Mirel V, Berdea P, Baciu C (2007) Tritium in water electrolytic enrichment and liquid scintillation counting. Radiat Meas. https://doi.org/10.1016/j.radmeas.2006.07.010
Boley NP, De Bièvre P, Taylor PDP, Uldall TA (2001) Requirements vs acceptability in proficiency testing schemes and other interlaboratory comparisons. Accred Qual Assur. https://doi.org/10.1007/s007690100331
Mazur J, Krzysztof K, Grządziel D, Guguła S, Mroczek M, Kozłowska B, Walencik-Łata A, Podgórska Z, Wołoszczuk K, Przylibski TA, Kowalska A, Domin E, Wysocka M, Chałupnik S, Chmielewska I, Długosz-Lisiecka M, Szajerski P, Chau ND, Krakowska P, Pliszczyński T, Ośko J, Dymecka M, Mazurek D (2020) National comparison of methods for determination of radon in water. Nukleonika. https://doi.org/10.2478/nuka-2020-0011
PROCORAD Intercomparison campaign plan 2023. http://www.procorad.org/media/etbfjiwr/a12-ind8-campaign_plan__2023-gb.pdf. Accessed 5 Sep 2023
ISO/FDIS 9698:2019 (E) (2019) Water quality - Tritium - Test method using liquid scintillation counting. International Organization for Standarization, Geneva
Zakowski K, Narozny M, Szocinski M, Darowicki K (2014) Influence of water salinity on corrosion risk - the case of the southern Baltic Sea coast. Environ Monit Assess. https://doi.org/10.1007/s10661-014-3744-3
ISO 9697:1992 Water quality – Measurement of gross beta activity in non-saline water. International Organization for Standarization, Geneva
Annual Report of Central Laboratory for Radiological Protection (CLOR). Measurements of radioactive contamination in samples of water, bottom sediments and fish in the framework of the monitoring of radioactive contamination of the Baltic Sea. https://www.clor.waw.pl/images/pliki/Raporty_roczne/Raport_roczny_2020.pdf. Accessed 5 Sept 2023
Schmied S, Zalewska T, Jakobson E (2018) Radionuclides in Seawater. In: 2018 Thematic Assessment of Radioactive Substances in the Baltic Sea 2011–2015, Baltic Sea Environment Proceedings No 151. HELCOM, Helsinki
Radwan I, Pietrzak-Flis Z, Wardaszko T (2001) Tritium in surface waters, tap water and precipitation in Poland during the 1994–1999 period. J Radioanal Nucl Chem. https://doi.org/10.1023/A:1006775300812
Duliński M, Różański K, Pierchała A, Gorczyca Z, Marzec M (2019) Isotopic composition of precipitation in Poland: a 44-year record. Acta Geophys. https://doi.org/10.1007/s11600-019-00367-2
Merešová D, Hanslík E, Juranová E, Sedlářová B (2017) Determination of low-level tritium concentrations in surface water and precipitation in Czech Republic. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-017-5410-z
Funding
Financial support was not provided for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no competing interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dymecka, M., Szaciłowski, G., Rzemek, K. et al. Low-level tritium measurements in freshwater and seawater samples. J Radioanal Nucl Chem 333, 3511–3522 (2024). https://doi.org/10.1007/s10967-023-09295-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09295-4