Abstract
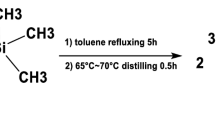
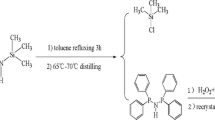
Tetraphenyldithiodiphosphonate is synthesized by polymerization precipitation. The P = S bond of the material has strong polarity, which is conducive to improving the electron mobility of the complex and inhibiting the accumulation between molecules. The synthesized tetraphenyldithiodiphosphonate is characterized by FT-IR, SEM, XPS and XRD. In addition, the pseudo-second-order kinetic model is used to simulate the experimental data well, and the adsorption process conforms to the Langmuir isotherm model. Thermodynamic studies of adsorption have shown that the adsorption process is essentially a spontaneous endothermic process. At room temperature, tetraphenyldithiodiphosphonate demonstrates a maximum adsorption capacity for uranium(VI) reaching 398.8 mg g−1.
















Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Dahake R, Tiwari P, Bansiwal A (2021) Multicycle adsorption and desorption for recovery of U(VI) from aqueous solution using oxime modified zeolite-A. J Radioanal Nucl Chem 327:133–142
Xie X, Wang Y, Xiong Z, Li H, Yao C (2020) Highly efficient removal of uranium(VI) from aqueous solution using poly(cyclotriphosphazene-co-polyethyleneimine) microspheres. J Radioanal Nucl Chem 326:1867–1877
Lv Z, Wang H, Chen C, Yang S, Chen L, Alsaedi A, Hayat T (2019) Enhanced removal of uranium(VI) from aqueous solution by a novel Mg-MOF-74-derived porous MgO/carbon adsorbent. J Colloid Interface Sci 537:A1–A10
Zhu K, Chen C, Xu M, Chen K, Tan X, Wakeel M, Alharbi NS (2018) In situ carbothermal reduction synthesis of Fe nanocrystals embedded into N-doped carbon nanospheres for highly efficient U(VI) adsorption and reduction. Chem Eng J 331:395–405
Sun Y, Wang X, Ai Y, Yu Z, Huang W, Chen C, Wan X (2017) Interaction of sulfonated graphene oxide with U(VI) studied by spectroscopic analysis and theoretical calculations. Chem Eng J 310:292–299
Liu X, Xu X, Sun J, Alsaed A, Hayat T, Li J, Wang X (2018) Insight into the impact of interaction between attapulgite and graphene oxide on the adsorption of U(VI). Chem Eng J 343:217–224
Han M, Kong L, Hu X, Chen D, Xiong X, Zhang H, Ruan Y (2018) Phase migration and transformation of uranium in mineralized immobilization by wasted bio-hydroxyapatite. J Clean Prod 197:886–894
Tatarchuk T, Shyichuk A, Mironyuk I, Naushad M (2019) A review on removal of uranium(VI) ions using titanium dioxide based sorbents. J Mol Liq 293:111563
Mei D, Liu L, Yan B (2023) Adsorption of uranium(VI) by metal-organic frameworks and covalent-organic frameworks from water. Coord Chem Rev 475:214917
Tang M, Chen J, Wang P, Wang C, Ao Y (2018) Highly efficient adsorption of uranium(VI) from aqueous solution by a novel adsorbent: titanium phosphate nanotubes. Environ Sci Nano 5(10):2304–2314
Prusty S, Somu P, Sahoo JK, Panda D, Sahoo SK, Rao KS (2022) Adsorptive sequestration of noxious uranium(VI) from water resources: a comprehensive review. Chemosphere 308:136278
Wang XL, Li Y, Huang J, Zhou YZ, Li BL, Liu DB (2019) Efficiency and mechanism of adsorption of low concentration uranium in water by extracellular polymeric substances. J Environ Radioact 197:81–89
Danko B, Dybczyński RS, Samczyński Z, Gajda D, Herdzik-Koniecko I, Zakrzewska-Kołtuniewicz G, Kulisa K (2017) Ion exchange investigation for recovery of uranium from acidic pregnant leach solutions. Nukleonika 62(3):213–221
Chen L, Zhao D, Chen S, Wang X, Chen C (2016) One-step fabrication of amino functionalized magnetic graphene oxide composite for uranium(VI) removal. J Colloid Interface Sci 472:99–107
Masakazu K, Nicolas P, Wooyong U, Jon C, O’Day Peggy A (2014) Influence of phosphate and silica on U(VI) precipitation from acidic and neutralized wastewaters. Environ Sci Technol 48(11):6097–6610
Zhou L, Wang Y, Zou H, Liang X, Zeng K, Liu Z, Adesina AA (2016) Biosorption characteristics of uranium(VI) and thorium(IV) ions from aqueous solution using CaCl2-modified Giant Kelp biomass. J Radioanal Nucl Chem 307(1):635–644
Khawassek YM, Masoud AM, Taha MH, Hussein AEM (2018) Kinetics and thermodynamics of uranium ion adsorption from waste solution using Amberjet 1200 H as cation exchanger. J Radioanal Nucl Chem 315:493–502
Saleh TA, Tuzen M, Sarı A (2017) Polyethylenimine modified activated carbon as novel magnetic adsorbent for the removal of uranium from aqueous solution. Chem Eng Res Des 117:218–227
Wang Z, Huang FY, Liu Y, Yi FC, Feng Y, Luo Y, Wang ZY (2022) Adsorption properties and mechanism of uranium by three biomass materials. Radiochim Acta 110(1):23–35
Amesh P, Venkatesan KA, Suneesh AS, Gupta DK, Ravindran TR (2021) Adsorption of uranium by diethylenetriamine functionalized magnetic mesoporous silica. Environ Nanotechnol Monit Manag 16:100583
Sun Z, Chen D, Chen B, Kong L, Su M (2018) Enhanced uranium(VI) adsorption by chitosan modified phosphate rock. Colloid Surf A 547:141–147
Tan J, Wang Y, Liu N, Liu M (2018) Adsorption of uranium(VI) from aqueous solution by tetraphenylimidodiphosphinate. J Radioanal Nucl Chem 315:119–126
Ma Z, Wang Y, Liu M, Luo Y, Xie X, Xiong Z (2019) Highly efficient uranium(VI) removal from aqueous solution using poly(cyclotriphosphazene-co-4,4′-diaminodiphenyl-ether) crosslinked microspheres. J Radioanal Nucl Chem 321:1093–1107
Kou Y, Zhang L, Liu B, Zhu L, Duan T (2020) Phosphonate modified MoS2 composite material for effective adsorption of uranium(VI) in aqueous solution. J Radioanal Nucl Chem 323:641–649
Ye T, Liu Z, Cai Z (2020) Adsorption of uranium(VI) from aqueous solution by novel dibutyl imide chelating resin. J Radioanal Nucl Chem 323(1):223–232
Onche EU (2019) Soft processing for the introduction of metal dopants to metal sulfide semiconductor thin films and nanoparticles. The University of Manchester (United Kingdom)
Oyetunde TT (2011) Novel precursors for chalcogenide materials. The University of Manchester (United Kingdom)
Zafar S, Khalid N, Mirza ML (2015) Sequestering of thorium ions from aqueous media on rice husk: equilibrium, kinetic and thermodynamic studies. Radiochim Acta 103(5):385–395
Ouyang Y, Zhao L, Deng M, Yang P, Peng G (2023) Preparation of diethylenetriamine-functionalized thiosulfate intercalated ZnNiAl-LDHs and its removal behavior and mechanism of U(VI). Chem Eng J 452:139486
Guan Y, Mao YH, Wei D, Wang XX, Zhu PX (2013) Adsorption thermodynamics and kinetics of disperse dye on poly (p-phenylene benzobisoxazole) fiber pretreated with polyphosphoric acid. Korean J Chem Eng 30:1810–1818
Kaynar UH, Şabikoğlu İ (2018) Adsorption of thorium(IV) by amorphous silica; response surface modelling and optimization. J Radioanal Nucl Chem 318:823–834
Zhong X, Sun Y, Zhang Z, Dai Y, Wang Y, Liu Y, Liu Y (2019) A new hydrothermal cross-linking ion-imprinted chitosan for high-efficiency uranium removal. J Radioanal Nucl Chem 322(2):901–911
Niu CP, Zhang CR, Liu X, Liang RP, Qiu JD (2023) Synthesis of propenone-linked covalent organic frameworks via Claisen–Schmidt reaction for photocatalytic removal of uranium. Nat Commun 14(1):4420
Rahman N, Varshney P, Nasir M (2021) Synthesis and characterization of polydopamine/hydrous zirconium oxide composite and its efficiency for the removal of uranium(VI) from water. Environ Nanotechnol Monit Manag 15:100458
Younes A, Ali JS, Duda A, Alliot C, Huclier-Markai S, Wang J, Alexandratos SD (2021) Uptake and removal of uranium by and from human teeth. Chem Res Toxicol 34(3):880–891
Asgari Fard Z, Sobhani A, Monsef R, Salavati-Niasari M (2019) Lead carbonate hydroxide nanostructures: a new hydrothermal synthesis in the presence of ethylenediamine and hydrazine and investigation photocatalytic behavior. J Mater Sci Mater Electron 30:20947–20957
Liu P, Yu Q, Zhang X, Chen J, Xue Y, Ma F (2021) Removal of U(VI) from aqueous solution using AO-artificial zeolite. J Radioanal Nucl Chem 327:39–47
Pengfei Y, Yuanxin X, Na Y, Yong A (2020) Preparation of uniform highly dispersed Mg–Al-LDHs and their adsorption performance for chloride ions. Ind Eng Chem Res 59(22):10697–10704
Acknowledgements
We are grateful to the anonymous reviewers for their constructive comments. The authors gratefully acknowledge support from the National Natural Science Foundation of China (51803088), Scientific Research Fund of Hunan Education Department (17C1360).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Z., Wang, Y., Xu, Y. et al. Adsorption of uranium(VI) in aqueous solution by tetraphenyldithiodiphosphonate. J Radioanal Nucl Chem 333, 357–373 (2024). https://doi.org/10.1007/s10967-023-09266-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09266-9