Abstract
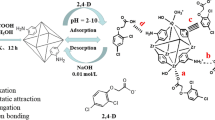
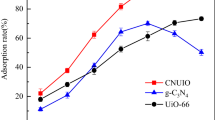
In this study, a crystal material UIO-66-NH2-BPDA was designed by grafting BPDA onto UIO-66-NH2 and applied to extract U(VI) from aqueous solution. According to performance tests, UIO-66-NH2-BPDA exhibits excellent chemical and thermal stability. The experimental results show that U(VI) adsorption by UIO-66-NH2-BPDA was tested to be 346.58 mg g−1 at pH = 4 and T = 298 K. The data simulation demonstrates that the adsorption process was consistent with Langmuir isotherm and pseudo-second-order model. Meanwhile, the thermodynamic parameters suggest that the adsorption process was spontaneous and endothermic. Most noteworthy is the good selectivity for U(VI) adsorption by UIO-66-NH2-BPDA. It can be seen that UIO-66-NH2-BPDA can be used as a potential effective adsorbent to remove U(VI) from wastewater.










Similar content being viewed by others
References
Bai Z-H, Liu Q, Zhang H-S, Liu J-Y, Yu J, Wang J (2020) A novel 3D reticular anti-fouling bio-adsorbent for uranium extraction from seawater: polyethylenimine and guanidyl functionalized hemp fibers. Chem Eng J 382:122555
Zhang N, Yuan L-Y, Guo W-L, Luo S-Z, Chai Z-F, Shi W-Q (2017) Extending the use of highly porous and functionalized MOFs to Th (IV) capture. ACS Appl Mater Interfaces 9(30):25216–25224
Mollick S, Saurabh S, More YD, Fajal S, Shirolkar MM, Mandal W, Ghosh SK (2022) Benchmark uranium extraction from seawater using an ionic macroporous metal–organic framework. Energy Environ Sci 15(8):3462–3469
Wang J-L, Zhuang S-T (2019) Extraction and adsorption of U (VI) from aqueous solution using affinity ligand-based technologies: an overview. Rev Environ Sci Bio/Technol 18:437–452
Lv S-Y, Li M, Wu X-Y, Zhang X-W, Hua Y-L, Bi L, Fang Q, Cai T (2021) A non-polluting method for rapidly purifying uranium-containing wastewater and efficiently recovering uranium through electrochemical mineralization and oxidative roasting. J Hazard Mater 416:125885
Zhang Z, Zhang D, Shi C, Liu W, Chen L-H, Miao Y, Diwu J, Li J-L, Wang S-A (2019) 3, 4-Hydroxypyridinone-modified carbon quantum dot as a highly sensitive and selective fluorescent probe for the rapid detection of uranyl ions. Environ Sci Nano 6(5):1457–1465
Singh S, Kaur M, Bajwa B, Kaur I (2022) Salicylaldehyde and 3-hydroxybenzoic acid grafted NH2-MCM-41: synthesis, characterization and application as U (VI) scavenging adsorbents using batch mode, column and membrane systems. J Mol Liq 346:117061
El-Din AFT, Elshehy EA, El-Khouly ME (2018) Cellulose acetate/EDTA-chelator assisted synthesis of ordered mesoporous HAp microspheres for efficient removal of radioactive species from seawater. J Environ Chem Eng 6(5):5845–5854
Wang Z, Hu H-M, Huang L-Q, Lin F-Y, Liu S, Wu T, Alharbi NS, Rabah SO, Lu Y-X, Wang X-K (2020) Graphene aerogel capsulated precipitants for high efficiency and rapid elimination of uranium from water. Chem Eng J 396:125272
Shakur H, Saraee KRE, Abdi M, Azimi G (2016) Highly selective and effective removal of uranium from contaminated drinking water using a novel PAN/AgX/ZnO nanocomposite. Microporous Mesoporous Mater 234:257–266
Ang KL, Li D, Nikoloski AN (2018) The effectiveness of ion exchange resins in separating uranium and thorium from rare earth elements in acidic aqueous sulfate media. Part 2. Chelating resins. Minerals Eng 123:8–15
Yu Q, Yuan Y, Wen J, Zhao X, Zhao S, Wang D, Li C, Wang X, Wang N (2019) A universally applicable strategy for construction of anti-biofouling adsorbents for enhanced uranium recovery from seawater. Adv Sci 6(13):1900002
Mei D, Li H, Liu L, Jiang L, Zhang C, Wu X, Dong H, Ma F (2021) Efficient uranium adsorbent with antimicrobial function: oxime functionalized ZIF-90. Chem Eng J 425:130468
Shu Y-Z, Xie J-X, Cheng C-H, Chen L-Y, Guo K-X, Peng G-W (2022) Bio-enrichment of heavy metals U (VI) in wastewater by protein DSR A. World J Microbiol Biotechnol 38(10):174
Cheng C-H, Xie J-X, Zhu Q-Q, Chen L-Y, Guo K-X, Li S-S, He S-Y, Xiao F-Z (2021) The reduction effect and mechanism of Deinococcus radiodurans transformed dsrA gene to uranyl ions. J Radioanal Nucl Chem 330:1075–1090
Dai Z-R, Zhen Y, Sun Y-S, Li L, Ding D-X (2021) ZnFe2O4/g-C3N4 S-scheme photocatalyst with enhanced adsorption and photocatalytic activity for uranium (VI) removal. Chem Eng J 415:129002
Cao X, Yu K-F, Zhang Y, Li N, Wang P, Zhou L, Gong X, Wang H-B, Yang F, Zhu W-K (2022) Efficient strategy for U (VI) photoreduction: simultaneous construction of U (VI) confinement sites and water oxidation sites. ACS Appl Mater Interfaces 15(1):1063–1072
Lam ITY, Yuan Y, Bang K-T, Choi S-J, Shin D-M, Lu D, Kim Y (2023) Towards the fastest kinetics and highest uptake of post-functionalized UiO-66 for Hg2+ removal from water. Nanoscale 15(25):10558–10566
Yang W-T, Pan Q-H, Song S-Y, Zhang H-J (2019) Metal–organic framework-based materials for the recovery of uranium from aqueous solutions. Inorganic Chem Front 6(8):1924–1937
Cheang T, Huang W-B, Li W-P, Ren S-Y, Wen H-H, Zhou T, Zhang Y-C, Lin W-H (2022) Exposed carboxyl functionalized MIL-101 derivatives for rapid and efficient extraction of heavy metals from aqueous solution. Colloids Surf A 649:129517
Li S-Q, Chen Y-F, Pei XK, Zhang S-H, Feng X, Zhou J-W, Wang B (2016) Water purification: adsorption over metal-organic frameworks. Chin J Chem 34(2):175–185
Abuçafy MP, da Silva BL, Oshiro-Junior JA, Manaia EB, Chiari-Andréo BG, Armando RA, Frem RC, Chiavacci LA (2020) Advances in the use of MOFs for cancer diagnosis and treatment: an overview. Curr Pharm Des 26(33):4174–4184
Luo Z-D, Fan S-R, Gu C-Y, Liu W-C, Chen J-X, Li B-H, Liu J-Q (2019) Metal–organic framework (MOF)-based nanomaterials for biomedical applications. Curr Med Chem 26(18):3341–3369
Li S, Han W-Y, An QF, Yong K-T, Yin M-J (2023) Defect Engineering of MOF‐Based Membrane for Gas Separation. Adv Funct Mater, 2303447.
Daniel M, Mathew G, Anpo M, Neppolian B (2022) MOF based electrochemical sensors for the detection of physiologically relevant biomolecules: an overview. Coord Chem Rev 468:214627
Ma L, Gao J, Huang C, Xu X, Xu L, Ding R-H, Bao H-L, Wang Z-Q, Xu G, Li Q-G (2021) UiO-66-NH-(AO) MOFs with a new ligand BDC-NH-(CN) for efficient extraction of uranium from seawater. ACS Appl Mater Interfaces 13(48):57831–57840
Mohammadi L, Hosseinifard M, Vaezi MR (2023) Stabilization of palladium-nanoparticle-decorated postsynthesis-modified Zr-UiO-66 MOF as a reusable heterogeneous catalyst in C-C coupling reaction. ACS Omega 8(9):8505–8518
Zhao Z-W, Cheng G, Zhang Y-Z, Han B, Wang X-K (2021) Metal-organic-framework based functional materials for uranium recovery: performance optimization and structure/functionality-activity relationships. ChemPlusChem 86(8):1177–1192
Carboni M, Abney CW, Liu SB, Lin WB (2013) Highly porous and stable metal–organic frameworks for uranium extraction. Chem Sci 4(6):2396–2402
Zhang L, Wang L-L, Feng X-F, Luo M-B, Luo F (2016) Coumarin-modified microporous-mesoporous Zn-MOF-74 showing ultra-high uptake capacity and photo-switched storage/release of UVI ions. J Hazard Mater 311:30–36
Chen L, Bai Z-L, Zhu L, Zhang L-J, Cai Y-W, Li Y-X, Liu W, Wang Y-L, Chen L-H, Diwu J (2017) Ultrafast and efficient extraction of uranium from seawater using an amidoxime appended metal–organic framework. ACS Appl Mater Interfaces 9(38):32446–32451
Yang Y, Li J-L, Sheng D-L, Ma Q, Zhang Y-Y (2023) Preparation of EDTA-modified UiO-66 for the selective removal of Cu (II) from water. J Solid State Chem 324:124063
Tripathi S, Sreenivasulu B, Suresh A, Rao CB, Sivaraman N (2020) Assorted functionality-appended UiO-66-NH2 for highly efficient uranium (vi) sorption at acidic/neutral/basic pH. RSC Adv 10(25):14650–14661
Xiong J, Hu S, Liu Y, Yu J, Yu H-Z, Xie L, Wen J, Wang X-L (2017) Polypropylene modified with amidoxime/carboxyl groups in separating uranium (VI) from thorium (IV) in aqueous solutions. ACS Sustain Chem Eng 5(2):1924–1930
Wang Y, Lin Z-W, Zhu J-H, Liu J-Y, Yu J, Liu Q, Chen R-R, Li Y, Wang J (2022) Co-construction of molecular-level uranyl-specific “nano-holes” with amidoxime and amino groups on natural bamboo strips for specifically capturing uranium from seawater. J Hazard Mater 437:129407
Luan X-F, Wang C-Z, Wu Q-Y, Lan J-H, Chai Z-F, Xia L-S, Shi W-Q (2022) Theoretical insights into selective extraction of uranium from seawater with tetradentate N, O-mixed donor ligands. Dalton Trans 51(30):11381–11389
Dean NE, Hancock RD, Cahill CL, Frisch M (2008) Affinity of the highly preorganized ligand PDA (1, 10-phenanthroline-2, 9-dicarboxylic acid) for large metal ions of higher charge. A crystallographic and thermodynamic study of PDA complexes of thorium (IV) and the uranyl (VI) ion. Inorganic chem 47(6):2000–2010.
Liu J-L, Wang X-B, Zhao Y-Y, Xu Y-F, Pan Y, Feng S-G, Liu J, Huang X-H, Wang H-T (2022) NH3 plasma functionalization of UiO-66-NH2 for highly enhanced selective fluorescence detection of u (vi) in water. Anal Chem 94(28):10091–10100
Zhang P, Chen Y-W, Chen Y-Z, Guo Q-Q, Liu Y-S, Yang Y, Cao Q, Chong H-B, Lin M-Z (2023) Functionalized hierarchically porous carbon doped boron nitride for multipurpose and efficient treatment of radioactive sewage. Sci Total Environ, 161378.
Du Y-R, Li X-Q, Lv X-J, Jia Q (2017) Highly sensitive and selective sensing of free bilirubin using metal–organic frameworks-based energy transfer process. ACS Appl Mater Interfaces 9(36):30925–30932
Gao Y, Yao L, Zhang S-Z, Yue Q-Y, Yin W-Y (2023) Versatile crosslinking synthesis of an EDTA-modified UiO-66-NH2/cotton fabric composite for simultaneous capture of heavy metals and dyes and efficient degradation of organophosphate. Environ Pollut 316:120622
Vellingiri K, Kumar P, Deep A, Kim K-H (2017) Metal-organic frameworks for the adsorption of gaseous toluene under ambient temperature and pressure. Chem Eng J 307:1116–1126
Zhang G, Fan H-M, Zhou R-Y, Yin W-Y, Wang R-B, Yang M, Xue Z-Y, Yang Y-S, Yu J-X (2022) Decorating UiO-66-NH2 crystals on recyclable fiber bearing polyamine and amidoxime bifunctional groups via cross-linking method with good stability for highly efficient capture of U (VI) from aqueous solution. J Hazard Mater 424:127273
Fu X-B, Liu J, Ren Z, Zhang S-Q, Xiao F-Z, Peng G-W (2022) Introduction of phosphate groups into metal-organic frameworks to synthesize MIL-101 (Cr)-PMIDA for selective adsorption of U (VI). J Radioanal Nucl Chem 331(2):889–902
Takao K, Bell TJ, Ikeda Y (2013) Actinide chemistry in ionic liquids. Inorg Chem 52(7):3459–3472
Wang C, Xiao F-Z, Pu Y-Q, Xu Y-L, Xu D-Y, Zhang K, Liu Y, Peng G-W (2018) Preparation of p-carboxyphenyl azo calix [4] arene phosphate derivative and its extraction properties toward uranium (VI). J Radioanal Nucl Chem 317:1235–1241
Wu J, Zhou J, Zhang S-W, Alsaedi A, Hayat T, Li J, Song Y-T (2019) Efficient removal of metal contaminants by EDTA modified MOF from aqueous solutions. J Colloid Interface Sci 555:403–412
Skodras G, Diamantopoulou I, Pantoleontos G, Sakellaropoulos G (2008) Kinetic studies of elemental mercury adsorption in activated carbon fixed bed reactor. J Hazard Mater 158(1):1–13
Vaddanam VS, Pamarthi A, Sengupta S, Sahoo M, Gupta SK, Balija S, Gopakumar G, Brahmananda Rao CVS, Suresh A, Jha SN (2023) Acid-Resistant Luminescent Si/UiO-66-Amidoxime (AO) Nanostructures for Rapid and Efficient Recovery of U (VI): Experimental and Theoretical Studies. ACS Appl Nano Mater 6 (10):8222–8237
Gao Q-H, Wang M-Ll, Zhao J-C, Zhang M-X, Shao D-d, Hu J-T, Wu G-Z (2022) Fabrication of amidoxime-appended UiO-66 for the efficient and rapid removal of U (VI) from aqueous solution. Microporous Mesoporous Mater 329:111511
Zhao B, Yuan L-Y, Wang Y, Duan T, Shi W-Q (2021) Carboxylated UiO-66 tailored for U (VI) and Eu (III) trapping: from batch adsorption to dynamic column separation. ACS Appl Mater Interfaces 13(14):16300–16308
Wang Y-L, Long J, Xu W-G, Luo H, Liu J, Zhang Y-P, Li J-C, Luo X-G (2021) Removal of uranium (VI) from simulated wastewater by a novel porous membrane based on crosslinked chitosan, UiO-66-NH2 and polyvinyl alcohol. J Radioanal Nucl Chem 328:397–410
Acknowledgements
The financial support from National Natural Science Foundation of China (No. 42377076) and the Hunan Provincial Natural Science Foundation Project of China (No. 2023JJ50129) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, X., Xu, D., Guo, S. et al. UIO-66-NH2 modified by BPDA for adsorption studies of U(VI). J Radioanal Nucl Chem 333, 531–544 (2024). https://doi.org/10.1007/s10967-023-09232-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09232-5