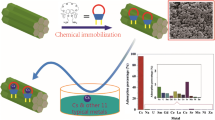
Abstract
The development of efficient and reusable Sr2+ adsorbent materials holds great significance for nuclear energy development and environmental protection. In order to address the low crown ether content in crown ether-based solid-phase adsorbents, this study employed condensation polymerization using di(aminobenzo)-18-crown-6 as monomers to prepare polymer materials and porous membranes. The adsorption kinetics, adsorption isotherm, and recyclability of the crown ether-based polymer were comprehensively investigated for Sr2+ adsorption. The results showed that the adsorption behavior of the polymer towards Sr2+ followed a pseudo-first-order kinetic model and Langmuir adsorption model, with a maximum adsorption capacity of 4.6 mg/g. Furthermore, even after five cycles, the adsorption performance of the polymer material for Sr2+ showed no significant decline. This study provides a new perspective for the development of long-lasting and highly efficient Sr2+ adsorbent materials.








Similar content being viewed by others
Data Availability
The data generated and analyzed in this study is available from corresponding author on reasonable request.
References
Zhang M, Gu P, Zhang Z, Liu J, Dong L, Zhang G (2018) Effective, rapid and selective adsorption of radioactive Sr2+ from aqueous solution by a novel metal sulfide adsorbent. Chem Eng J 351:668–677
Mu W, Yu Q, Gu J, Li X, Yang Y, Wei H, Peng S (2020) Bonding of crown ethers to α-zirconium phosphate—novel layered adsorbent for radioactive strontium separation. Sep Purif Technol 240:116658
Gao YJ, Feng ML, Zhang B, Wu ZF, Song Y, Huang XY (2018) An easily synthesized microporous framework material for the selective capture of radioactive Cs+ and Sr2+ ions. J Mater Chem A 6:3967–3976
Khani MH, Khamseh AG (2023) Statistical analysis, equilibrium and dynamic study on the biosorption of strontium ions on Chlorella vulgaris. J Radioanal Nucl Chem 332:3325–3334
Chaalal O, Zekri AY, Soliman AM (2015) A novel technique for the removal of strontium from water using thermophilic bacteria in a membrane reactor. J Ind Eng Chem 21:822–827
Prill B, Sedir U, Yusan S, Elmastas Gultekin O (2022) Strontium (II) biosorption studies on starch-functionalized magnetic nanobiocomposites using full factorial design method. J Polym Environ 30:5148–5162
Li WA, Peng YC, Ma W, Huang XY, Feng ML (2022) Rapid and selective removal of Cs+ and Sr2+ ions by two zeolite-type sulfides via ion exchange method. Chem Eng J 442:136377
İnan S (2022) Inorganic ion exchangers for strontium removal from radioactive waste: a review. J Radioanal Nucl Chem 331:1137–1154
Huang G, Jiang L, Shao L, Yang X, Huang J (2020) In situ electrochemical synthesis of Zn-Al layered double hydroxides for removal of strontium. Colloids Surfaces A Physicochem Eng Asp 597:124785
Cai YH, Yang XJ, Schäfer AI (2020) Removal of naturally occurring strontium by nanofiltration/reverse osmosis from groundwater. Membranes 10:1–23
Zhang L, Lu Y, Liu YL, Li M, Zhao HY, Hou LA (2016) High flux MWCNTs-interlinked GO hybrid membranes survived in cross-flow filtration for the treatment of strontium-containing wastewater. J Hazard Mater 320:187–193
Wang W, Luo J, Wei W, Liu S, He J, Ma J (2021) An asymmetric pulsed current-assisted electrochemical method for Sr(II) extraction using supramolecular composites. Chemosphere 271:129531
Alshuiael SM, Al-Ghouti MA (2022) Development of a novel tailored ion-imprinted polymer for recovery of lithium and strontium from reverse osmosis concentrated brine. Sep Purif Technol 295:121320
Kaveeshwar AR, Kumar PS, Revellame ED, Gang DD, Zappi ME, Subramaniam R (2018) Adsorption properties and mechanism of barium (II) and strontium (II) removal from fracking wastewater using pecan shell based activated carbon. J Clean Prod 193:1–13
Schön U, Blasius E, Klein W (1985) Separation of strontium from nuclear waste solutions by solvent extraction with crown ethers. Anim Reprod Sci 9:95–98
Chen Z, Wu Y, Wei Y (2014) Adsorption characteristics and radiation stability of a silica-based DtBuCH18C6 adsorbent for Sr(II) separation in HNO3 medium. J Radioanal Nucl Chem 299:485–491
Bai F, He C, Chen G, Wei J, Wang J, Ye G (2013) Synthesis of alkyl substituted dicyclohexano-18-crown-6 homologues for strontium extraction in HNO3 media. Energy Procedia 39:396–402
Pathak S, Jayabun S, Boda A, Ali SM, Sengupta A (2020) Experimental and theoretical insight into the extraction mechanism, kinetics, thermodynamics, complexation and radiolytic stability of novel calix crown ether in ionic liquid with Sr2+. J Mol Liq 316:113864
Momen MA, Dietz ML (2021) High-capacity extraction chromatographic materials based on polysulfone microcapsules for the separation and preconcentration of lanthanides from aqueous solution. React Funct Polym 160:612–621
Ma J, Zhang Y, Ouyang J, Wu X, Luo J, Liu S, Gong X (2019) A facile preparation of dicyclohexano-18-crown-6 ether impregnated titanate nanotubes for strontium removal from acidic solution. Solid State Sci 90:49–55
Yest TL, Fagan BC, Allain LR, Bames CE, Dai S, Sepaniak MJ, Xue Z (2000) Crown ether-doped sol-gel materials for strontium(II) separation. Anal Chem 72:5516–5519
Zhang A, Xiao C, Liu Y, Hu Q, Chen C, Kuraoka E (2010) Preparation of macroporous silica-based crown ether materials for strontium separation. J Porous Mater 17:153–161
Ye G, Bai F, Wei J, Wang J, Chen J (2012) Novel polysiloxane resin functionalized with dicyclohexano-18-crown-6 (DCH18C6): synthesis, characterization and extraction of Sr(II) in high acidity HNO3 medium. J Hazard Mater 225–226:8–14
Song Y, Du Y, Lv D, Ye G, Wang J (2014) Macrocyclic receptors immobilized to monodisperse porous polymer particles by chemical grafting and physical impregnation for strontium capture: a comparative study. J Hazard Mater 274:221–228
Liu Z, Zhou Y, Guo M, Lv B, Wu Z, Zhou W (2019) Experimental and theoretical investigations of Cs+ adsorption on crown ethers modified magnetic adsorbent. J Hazard Mater 371:712–720
Bai F, Ye G, Chen G, Wei J, Wang J, Chen J (2013) New macrocyclic ligand incorporated organosilicas: co-condensation synthesis, characterization and separation of strontium in simulated high level liquid waste. React Funct Polym 73:228–236
Ye G, Leng Y, Bai F, Wei J, Wang J, Chen J (2013) Site-selective functionalization of periodic mesoporous organosilica (PMO) with macrocyclic host for specific and reversible recognition of heavy metal. Chem Asian J 8:1482–1488
Pei H, Yan F, Ma X, Li X, Liu C, Li J, Cui Z, He B (2018) In situ one-pot formation of crown ether functionalized polysulfone membranes for highly efficient lithium isotope adsorptive separation. Eur Polym J 109:288–296
He J, Mao L, Ma X, Hua J, Cui Z, He B, Pei H, Li J (2022) Highly-efficient adsorptive separation of Cs+ from aqueous solutions by porous polyimide membrane containing dibenzo-18-crown-6. Sep Purif Technol 299:121757
Wei Y, Rakhatkyzy M, Salih KAM, Wang K, Hamza MF, Guibal E (2020) Controlled bi-functionalization of silica microbeads through grafting of amidoxime/methacrylic acid for Sr(II) enhanced sorption. Chem Eng J 402:125220
Nishiyama Y, Hanafusa T, Yamashita J, Yamamoto Y, Ono T (2016) Adsorption and removal of strontium in aqueous solution by synthetic hydroxyapatite. J Radioanal Nucl Chem 307:1279–1285
Ahmad AL, Abdulkarim AA, Ooi BS, Ismail S (2013) Recent development in additives modifications of polyethersulfone membrane for flux enhancement. Chem Eng J 223:246–267
Mousavi SM, Zadhoush A (2017) Investigation of the relation between viscoelastic properties of polysulfone solutions, phase inversion process and membrane morphology: the effect of solvent power. J Memb Sci 532:47–57
Sukitpaneenit P, Chung TS (2009) Molecular elucidation of morphology and mechanical properties of PVDF hollow fiber membranes from aspects of phase inversion, crystallization and rheology. J Memb Sci 340:192–205
Hadisaputra S, Pranowo HD, Armunanto R (2012) Extraction of strontium(ii) by crown ether: insights from density functional calculation. Indones J Chem 12:207–216
Horwitz EP, Dietz ML, Fisher DE (1991) Separation and preconcentration of strontium from biological, environmental, and nuclear waste samples by extraction chromatography using a crown ether. Anal Chem 63:522–525
Pei H, Yan F, Wang Z, Liu C, Hou S (2019) Polysulfone-graft-4-aminobenzo-15-crown-5-ether based tandem membrane chromatography for efficient adsorptive separation of lithium isotopes. J Chromatogr A 1602:206–216
Doǧan M, Alkan M, Türkyilmaz A, Özdemir Y (2004) Kinetics and mechanism of removal of methylene blue by adsorption onto perlite. J Hazard Mater 109:141–148
Torrejos REC, Nisola GM, Park MJ, Shon HK, Seo JG, Koo S, Chung WJ (2015) Synthesis and characterization of multi-walled carbon nanotubes-supported dibenzo-14-crown-4 ether with proton ionizable carboxyl sidearm as Li+ adsorbents. Chem Eng J 264:89–98
Wang X, Li Y, Li H, Yang C (2016) Chitosan membrane adsorber for low concentration copper ion removal. Carbohydr Polym 146:274–281
Yang L, Xiao H, Qian Y, Zhao X, Kong XY, Liu P, Xin W, Fu L, Jiang L, Wen L (2022) Bioinspired hierarchical porous membrane for efficient uranium extraction from seawater. Nat Sustain 5:71–80
Hong HJ, Ryu J, Park IS, Ryu T, Chung KS, Kim BG (2016) Investigation of the strontium (Sr(II)) adsorption of an alginate microsphere as a low-cost adsorbent for removal and recovery from seawater. J Environ Manag 165:263–270
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 22208196), the China postdoctoral science foundation (Grant No. 2021M702014), Natural Science Foundation of Shandong Province, China (Grant No. ZR202111080133).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that there is no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pei, H., Liu, J., Yue, M. et al. Investigation of the strontium (Sr(II)) adsorption of 18-crown-6 based polymer. J Radioanal Nucl Chem 332, 5051–5057 (2023). https://doi.org/10.1007/s10967-023-09163-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09163-1