Abstract
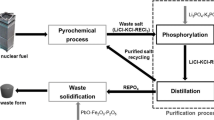
In the pyroprocessing of used nuclear fuel, it is difficult to recover U in a recyclable form from LiCl–KCl eutectic salt containing a high concentration of rare earths (REs) by co-deposition of U and RE. To derive a solution to this problem, it is necessary to develop a technology to reduce the RE concentration or separate the RE in the salt. To this end, an integrated process of chemical conversion of UCl3 into an oxide form and electro-deposition of NdCl3 in a metal form was evaluated using a simulated LiCl–KCl salt containing about 1.4 wt% of UCl3 and 4.0 wt% NdCl3 in this study. Through the integrated process, higher than 99% conversion efficiency of U was obtained and the concentration of Nd in the simulated salt was reduced to less than 1/100. It is expected that the integrated process also can be applied effectively to the recovery of U from LiCl–KCl eutectic salt containing a high concentration of RE.







Similar content being viewed by others
References
Lin J, Shen Y, Li X, Hasnaoui A (2021) BRICS carbon neutrality target: measuring the impact of electricity production from renewable energy sources and globalization. J Environ Manag 298:113460
Murakami T, Kato T, Rodrigues A, Ougier M, Iizuka M, Koyama T, Glatz J-P (2014) Anodic dissolution of irradiated metallic fuels in LiCl–KCl melt. J Nucl Mater 452:517–525
Riley BJ, Rieck BT, McCloy JS, Crum JV, Sundaram SK, Vienna JD (2012) Tellurite glass as a waste form for mixed alkali-chloride waste streams: candidate materials section and initial testing. J Nucl Mater 424:29–37
Murakami T, Rodrigues A, Ougier M, Iizuka M, Koyama T, Glatz J-P (2015) Actinides recovery from irradiated metallic fuel in LiCl–KCl melts. J Nucl Mater 466:502–508
Eun HC, Choi JH, Kim NY, Lee TK, Ham SY, Lee KR, Park HS, Ahn DH (2016) A reactive distillation process for the treatment of LiCl–KCl eutectic waste containing rare earth chlorides. J Nucl Mater 480:69–74
Soucek P, Malmbeck R, Mendes E, Nourry C, Glatz J-P (2010) Exhaustive electrolysis for recovery of actinides from molten LiCl–KCl using solid aluminum cathodes. J Radioanal Nucl Chem 286(3):823–828
Eun HC, Choi JH, Lee TK, Cho IH, Kim NY, Yu JU, Park HS, Ahn DH (2015) Separation characteristics of NdCl3 from LiCl–KCl eutectic salt in a reactive distillation process using Li2CO3 or K2CO3. J Nucl Fuel Cycle Waste Technol 13(3):181–186
Kang YH, Hwang SC, Lee HS, Kim EH, Park SW, Lee JH (2009) Effects of neodymium oxide on an electrorefining process of uranium. J Mater Process Technol 209:5008–5013
Lee HS, Park GI, Kang GH, Hur JM, Kim JG, Ahn DH, Cho YZ, Kim EH (2011) Pyproprocessig technology development at KAERI. Nucl Eng Technol 43(4):317–328
Ronie A (2002) Outokumpu HSC chemistry for windows, OutoKumpu Research. Pori, Finland
Killinger DP, Phongikaroon S (2020) Investigation of dissolution and removal of adhered LiCl–KCl–UCl3 salt from electrodeposited uranium dendrites using deionized waster, methanol, and ethanol. J Nucl Fuel Cycle Waste Technol 18(4):549–562
Acknowledgements
This research was funded by the Korea Atomic Energy Research Institute (Grant No. 521230-23, Republic of Korea)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eun, HC., Kim, TJ., Park, WS. et al. A method for the separation of U and RE from LiCl–KCl eutectic salt containing a high concentration of RE. J Radioanal Nucl Chem 332, 4567–4572 (2023). https://doi.org/10.1007/s10967-023-09160-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09160-4