Abstract
The main aim of this work was to develop a suitable sorbent for the separation and determination of 226Ra through 133Ba (radio tracer) in water samples using fly ash sorbent. After the modification with KMnO4 the effects of pH, competing ions, the possibility of elution, and the effect of water volume were tested. As a suitable eluent 6 mol/L HCl was chosen, while the sorbent worked best at pH 6–8. The developed method is advantageous for minimizing the time required for separation, the volume of chemicals used, and the waste generated after separation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, over 20 radium isotopes are known, among them four can be found naturally [1]. The naturally occurring isotope 226Ra is one of the most radiotoxic long-lived radionuclides present in the environment [2]. For this reason, national and international legislation requires its constant monitoring in the human environment, especially in drinking water. Therefore, for its determination, it is necessary to have reliable and accurate determination methods. There are a number of methods described in the literature for its determination, but it is somewhat common to use gamma spectrometry and/or radon emanation techniques for routine determinations. However, these techniques have some drawbacks, which can significantly affect the reliability and accuracy of the determination, such as the emanation of radon from the measuring vessel during the measurement and the follow-up measurement in conditions of radiochemical disequilibrium [3]. In addition, in gamma spectrometric measurement some interference can appear (i.e., from 235U using the line 186 keV). Other techniques, like thermal ionisation mass spectrometry, which has high analytical precision, requires extremely pure Ra load without presence of any Ba atoms. On the other hand, alpha-spectrometry in combination with simple and rapid radium isolation procedures can be advantageous over the mentioned techniques because measurements can be performed immediately after radium isolation from sample with significantly lower detection limits compared to other techniques [4]. Namely radium activity in the environmental samples is low so that almost all determination methods include preconcentration step before separation. It is well known that radium is inclined to the same behaviour as its chemically similar elements from the same group of the periodic table, particularly barium [5]. This fact Sill exploit in 1987, and proposed a method for the determination of 226Ra that differed from other used techniques at that time. The method included microfiltration of co-precipitated Ba(Ra)SO4, using a non-radium tracer—133Ba for recovery determination [2].
In case of radium preconcentration, resins impregnated with manganese dioxide (MnO2) can be used. It enables simple, rapid and efficient radium preconcentration from large volume liquid samples [6,7,8].
Fly ash, from coal burning, is grey coloured and contains many essential elements. The chemical composition of fly ash: silica SiO2 (60–65%), alumina Al2O3 (25–30%), magnetite Fe3O4 and ferric oxide Fe2O3 (6–15%) allows its use for the synthesis of zeolite, alum and precipitated silica. Many of its physical and chemical properties make this material suitable for use as an adsorbent [9].
Limited literature data on the efficiency of radium separation using fly ash and the fact that MnO2 is effective in supporting radium isolation motivated us to investigate the use of impregnated fly ash with potassium permanganate (KMnO4) as sorbent for 226Ra isolation. Therefore, the aim of this paper was to develop a cheap, easy to prepare, MnO2 impregnated fly ash, to characterise it and to show that it can be used as a sorbent for efficient radium isolation. In this paper Ra was not used just Ba. Radium and barium have the same chemistry, so it is used for optimisation of new Ra determination methods as a non-isotopic tracer.
Materials and methods
Materials
Fly ash (under the commercial name MICROSILICA—SIOXID from OFZ, a.s., Istebné, Slovakia), potassium permanganate (KMnO4, Lachema, Czech Republic) and deionized water were used for the preparation of the modified fly ash sorbent. 133Ba was used in the experiments as radioactive tracer. Hydrochloric acid (HCl, Slavus s.r.o., Slovak Republic) and sodium hydroxide (NaOH, Lachema, Czech Republic) were used to adjust the pH. Magnesium sulphate hexahydrate (MgSO4 · 6 H2O, Slavus s.r.o. Slovak Republic) and calcium nitrate tetrahydrate (Ca(NO3)2 · 4 H2O, Slavus s.r.o., Slovak Republic) were applied to test the effect of competitive cations during adsorption. Hydrochloric acid (HCl, Slavus s.r.o., Slovak Republic) was used to eluate 226Ra from the column. Barium chloride dihydrate (BaCl2 · 2 H2O, p.a., Slavus, Bratislava) and ammonium sulphate ((NH4)2SO4; Slavus, p.a., Bratislava) were used for the preparation of samples for the alpha measurement.
Radiometric analysis
133Ba activity in solutions was determined using Gamma ORTEC HPGe 20,109 P detector with the data processing software Gamma Vision-32bit, f. EG&G ORTEC (6% of relative efficiency for 133Ba determination). Calibration of the gamma detector was performed using certified gamma standards obtained from the Czech Metrology Institute. All applied counting geometries were calibrated by using a certified standard. 20 mL scintillation vials were used for batch and column experiments, where from 5 to 15 mL of solutions were analysed. All data were obtained with a measurement error of 2% for the counting time of 3600 s. This measurement error does not reflect other error sources. The minimum detectable activity MDA = 0,28 Bq and it was calculated according to Currie [10].
Methods
As it is visible on Fig. 1 to develop the suitable conditions for radium separation from water samples, different experiments were carried out under column conditions described in Experimental conditions.
Preparation of the sorbent
The fly ash used in this work was purchased from the commercial company OFZ, a.s. (Slovak Republic) under the trade name MICROSILICA—SIOXID. Most particles have a diameter in the range of (10–300) μm. The material has the following guaranteed composition: min. 85% silica, max. 1% calcium oxide, max. 2% sulphates, max. 2% alkali content, max. 0.3% chlorides and the loss by annealing at 750 °C is maximally 4%.
The modification of the fly ash was done using a KMnO4 solution [8, 11, 12]. The fly ash (10 g) was rinsed twice with deionized water to remove fine dust particles and drench in 0.5 L of 0.5 mol/L KMnO4 for 2 h at 80 °C. The suspension was then cooled to room temperature and filtrated through filter paper (Surface weight: 84 g/m2, retention range 8–12 μm, filtration time: 20 s). The modified fly ash was washed with deionized water and air dried at room temperature.
Kinetics of sorption
The radionuclide tracer 133Ba was used to test the possibility of sorption on unmodified and modified fly ash [13]. The sorption experiments were carried out in 15 mL centrifugation tubes containing 0.5 g of fly ash (dry weight, unmodified and modified) and 10 mL of 0.1 mg/L Ba2+ carrier solution with 133Ba tracer (70 Bq/L). The centrifugation tubes were shaken in a reciprocal shaker at room temperature for 1 min to 30 min. In total, 10 samples were prepared. The supernatants were collected using a centrifuge (Centrifuge Frontier™ FC5706 Mult, Ohaus GmbH, 4000 rpm for 5 min.) to separate the sorbent from a solution. The sorption rate was monitored by measuring 133Ba in the solution after a certain contact time (by counting on HPGe detector).
Column experimental conditions
For all the experiments column filled with 0.5 g of modified sorbent (BV = 5 mL; column diameter 1 cm) and preconditioned with 2 × 20 mL of deionized water was used. Model solutions contained 0.5 mg/mL of Ba2+ carrier and were spiked with 133Ba (70 Bq/L) and passed through the column at a flow rate of 0.4 mL/min. The effluents were collected into 20 mL vials and the activity of 133Ba was measured by HPGe detector. All experiments were carried out two times. These conditions were used for all the experiments unless stated otherwise.
Influence of the pH change
To test the influence of pH on the sorption of the 133Ba the modified fly ash sorbent was loaded onto the column. The pH of model samples was adjusted to various values in the interval from 1 to 10 with step 1 pH, using 0.1 mol/L hydrochloric acid (HCl) or 0.1 mol/L sodium hydroxide (NaOH). The adjusted model samples were loaded onto the column filled with the preconditioned modified sorbent. The effluents were collected into plastic vials and 133Ba was measured using HPGe detector.
Effect of the elution solution
To test the influence of elution solution on the sorption of 133Ba the model solution (pH = 6–7) was loaded onto the column. Barium was eluted from the column using different concentrations of HCl and HNO3, both in a range of 1 mol/L to 9 mol/L. The eluted sample was collected into a plastic vial and the 133Ba activity was measured as described before.
Effect of competitive cations
The experiments to study the effect of competitive cations on the sorption of barium were carried out according to the above-mentioned column system conditions. In this case the model samples contained Na+, K+, Ca2+, Mg2+ ions with different concentrations: Na+: 7 g/L–150 g/L, K+: 7 g/L–150 g/L, Ca2+: 7 g/L–70 g/L, Mg2+: 7 g/L–112 g/L. The model samples were loaded onto the columns and the effluent fractions were collected into plastic vials in which the activity of 133Ba was measured as described.
Effect of volume of contaminated water
To study the effect of contaminated water on the sorption of 133Ba, different volumes of model samples (1 L–50 L) were used. The model samples were loaded onto the big glass column (length 20 cm, diameter 3 cm) filled with 3 g of modified fly ash sorbent. The time frame for the separation depending on the volume was up to 5 days. We used an infusion set to continuously dose the sample onto the column. Barium was eluted form the column using 100 mL of 6 mol/L HCl and 133Ba was measured as described.
Results and discussion
Emami [14] showed, that the charge of the silica surface in fly ash plays a significant role in the adsorption of radium as does the pH of the solution. Hence, the increase in pH leads to an increase in surface charge and consequently enables stronger binding of the cation on the surface. Therefore, it was assumed that radium can be separated using clean fly ash without any modification. However, obtained results showed that the unmodified sorbent has low sorption capacity for radium. In our previous paper was shown that sorption capacity of the fly ash can be increased after its treatment with suitable modifying agent [15] like manganese oxides, which show remarkable adsorbing abilities [8, 11, 12]. The treatment of fly ash with 0.5 mol/L KMnO4, resulted in capacity increase from 1.5 mg/L to 20 mg/L.
Kinetics of sorption
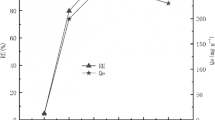
Sorption kinetics was examined by monitoring of barium sorption rate on modified sorbent as described in experimental part. From the results shown in Fig. 2, it can be seen that after 10 min percentage of barium sorption R has reached the maximum value and it can be assumed that the equilibrium has been reached. The sorption is faster in a first few minutes probably due to mostly non-occupied bind spots. In the later stages, the sorption is noticeably slower, since most of the free spots were already occupied, and there was a reduced amount of available free spots. This leads to the existence of a plateau area, where an equilibrium state is achieved between the two phases (liquid phase and solid sorbent) for the precise activity of 226Ra (amount of Ra2+ ions) in the sorbate. This concept was also suggested by other authors [8, 16], while researching the sorption of Ba2+ (chemical equivalent of Ra2+ ions) on manganese dioxide and indicated that an equilibrium will be achieved in 30 min.
Varga [8] tested the radium sorption at two different pH values (6 and 8). He obtained significant difference between the adsorption at these two pH values and concluded that the primary parameter that affects the sorption is exactly the pH. At pH = 8 the equilibrium was reached under 30 min. In our case the kinetics were tested at pH (7–8), and the time required for complete sorption was less than 10 min. These results are in agreement with those of Varga [8]. Later it is shown that the optimum working pH range for our sorbent is between 6 and 9.
Effect of the elution solution
Barium preconcentration can be achieved by elution of 133Ba using a suitable eluent. For the testing of the barium elution, two acids were used: HCl and HNO3. From the results shown in Fig. 3, it seems that hydrochloric acid is more suitable for the elution. Obtained results for barium yield showed that nitric acid gave lower yield compared with 6 mol/L HCl hydrochloric acid, (85 ± 11) % vs (97 ± 14) %. Therefore 6 mol/L concentration of HCl was used in the next experiments.
Effect of competitive cations
Natural water contains a significant number of dissolved cations and anions which can compete with the Ra2+ ions and limits the sorbent sorption potential as well as capacity for barium therefore also radium. The influence of the concentration of other ions on the sorption of barium was examined. Na+ and K+ ions do not affect barium sorption. However, from the results shown in Fig. 4, it can be seen that the presence of Ca2+ ions can significantly reduce the sorption yield. Up to a concentration of 50 g/L it was above 90%, but above this concentration, the sorption yield decreased rapidly.
Similar effect is caused by Mg2+, the sorption yield was higher than 90% up to a concentration of 70 g/L but above this concentration it decreases rapidly.
There are many articles in which researchers use fly ash in different forms or with different modifications as sorbent for barium separation. In one of them [17], fly ash was used for the removal of environmental pollutants. For the removal of barium, the mixture of 25 kg of sand and 2 kg of zeolite was used. The most interesting thing about the samples that came from the coal mine was their high content of different salts, up to 110 g/L. One hundred and twenty litres of salt water were tested. The water sample contained 49 700 mg/L Na+, 4 410 mg/L Mg2+ and 2 970 mg/L Ca2+. Other ions were present just in a negligible amount. When comparing the results with the modified fly ash used in this work, it is clear that the limits of the fly ash were much higher than the values determined in the coal mine water.
Influence of the pH change
The reason for studying the sorption of 226Ra (133Ba) on KMnO4 as a function of pH is, that the pH of the solutions can change the surface charge of oxides/hydroxides of metals. To adjust the pH value of the solutions, usually NaOH and HCl are used. The pH of the solutions can change in different ways, as has already been shown with environmental waters. Therefore, Koulouris [16] tested the adjustment of pH in distilled water enriched with 226Ra (133Ba) using NaOH, Na2CO3, and also HCl. According to the results, the use of different solutions to adjust the pH did not have any influence on the sorption.
In case of natural waters with pH from 7.5 to 8.5 and higher, a quantitative pre-concentration can be achieved without adjusting the pH, although absorption for radium is highly sensitive to pH. This factor should be considered when optimizing sample preparation step, as mentioned in Varga [8].
Varga [8] confirmed, that KMnO4 modified sorbents show a higher ability to bind radium from alkaline solutions, mainly from solutions with pH > 8.0. KMnO4 modified AG 1-X4 anion exchange sorbent showed similar sorption properties at different pH values as different KMnO4 modified sorbents [12, 18]. Koulouris [16] determined a wide spectrum of pH (3.6–12.0) while using MnO2 modified sorbents with particle size (0.60–1.20) mm. With values of pH < 3.6, he observed a rapid decrease in sorption. Because the plateau of the curve goes below 5.5, the author suggested that ion exchange might not be the main sorption process. This statement was also supported by kinetics testing, which gave an enthalpy change value of − 20.0 kJ/mol, significantly higher than the ion exchange process or the physical sorption process.
In our experiments, it was found that the sorption on the modified fly ash sorbent was limited until pH = 4.7 as are shown in Fig. 5. However, over pH = 5, the sorption yield was more than 90%. At pH > 9, the sorption yields decreased rapidly. The minimum value of pH, where the sorption yield was greater than 90%, is 5.
Effect of volume
The ability to separate radium directly from large volumes of water samples with minimal pre-treatment would lead to a significant reduction in process time in comparison to other alternative separation techniques. The resulting high capacity for samples would be advantageous in some applications, including routine monitoring of drinking water samples and compliance testing of outlets with regulatory goals [19]. The experiments were carried out under column conditions, where 1 g of modified fly ash sorbent was used as a sorption material. The water samples containing 133Ba tracer radionuclide, had volumes of 5 L, 10 L, 20 L, 30 L, 40 L, and 50 L and were loaded into the column. It was found that the sorption yield was higher than 92% at volumes from 5 to 30 L. A significant decrease in the sorption yield of 133Ba was observed after applying 40 L of water. All results are shown in Fig. 6.
Chałupnik et al. [20] a synthetic zeolite-type NaP1 sorbent in their work used, which was created from fly ash. For column experiments, a mixture of zeolite, sand, and gravel was used. Different types of water samples were tested: drinking water, barium containing water and two types of mine water. The barium removal efficiency from water exceeded 90% for all the water samples, except the water containing barium. In that case, after overloading the filter bed, the efficiency decreased significantly. During these experiments, leaching from the column of the very fine particles of zeolites was the biggest problem. According to the authors, this method must meet many specific requirements of the mining industry, since underground mine waste water can clog filters very effectively.
It seems that the application of zeolite materials together with fly ash for the construction of passive barriers will be a suitable solution to this problem. This material must be properly disposed because of the increased radioactivity in mine waters. To prevent any further radiation of miners, reuse is not expected. Because of this reason, any kind of application of ion exchangers, nanofibers, or polymers is excluded in the mining industry, mainly due to costs.
After the examination of results, it is visible that synthetic zeolites, and therefore fly ash, are suitable for the removal of barium and therefore radium from different contaminated waters. Moreover, it is suitable for single use, mainly because of its low cost and availability. From the results, it is clear that the method used in this paper is applicable only for samples with a volume less than 30 L per 1 g of modified fly ash sorbent.
Conclusion
The research carried out in this work was focused on the preparation of a sorbent for the separation and determination of 226Ra. The goal was to modify the fly ash using KMnO4 solution, in order to obtain higher capacity, to reduce the use of chemicals and to make the separation process easier, even for larger sample volumes. When testing the influence of volume of the model samples, it was found that a significant reduction in 133Ba sorption yield was observed when up to 40 L of contaminated water was used.
The developed and optimized method for the determination of 226Ra on modified fly ash is characterized by high capacity. Compared to other types of sorbents, this sorbent is affordable and cost effective. Another advantage of fly ash is that it does not have to be manufactured, but is a by-product of burning coal, and it is available worldwide. Fly ash is considered waste material. During the laboratory application itself, where chromatographic columns are used, it was easy to work with fly ash and the column did not clog even with larger volumes of water samples. The method developed has the advantage of minimizing the time required for separation, the volume of chemicals used, and the waste generated after separation. It could be used for the determination of 226Ra in samples of natural and mineral waters.
References
Medley P, Bollhöfer A, Iles M, Ryan B, Martin P (2005) Barium sulphate method for Radium – 226 analysis by alpha spectrometry. Internal Report 501, Supervising Medley P, Bollhöfer A, Iles M, Ryan B, Martin P (2005) Barium Sulphate Method for Radium – 226 analysis by alpha spectrometry. Internal Report 501, Supervising Scientist, Darwin. Unpublished paper
Sill CW (1987) Determination of Radium – 226 in ores, nuclear waste and environmental samples by high – resolution alpha spectrometry. Nucl Chem Waste Manag 7:239–256. https://doi.org/10.1016/0191-815X(87)90069-6
Maxwell SL, Culligan BK (2012) Rapid determination of 226Ra in environmental samples. J Radioanal Nucl Chem 293:149–156. https://doi.org/10.1007/s10967-012-1627-z
Lawrie WC, Desmond JA, Spence D, Anderson S, Edmondson C (2000) Determination of Radium – 226 in environmental and personal monitoring samples. Appl Radiat Isot 53:133–137. https://doi.org/10.1016/S0969-8043(00)00168-8
Kirby HW, Salutsky ML (1964) The Radiochemistry of Radium. National Academy of Sciences – National Research Council, Nuclear Science series. USA
Dietz ML, Horwitz EP, Chiarizia R, Bartsch R (1998) Process for separation and preconcentration of radium from water. World Intellectual Property Organization, WO 98/55201
Abbasi A (2018) A review of the analytical methodology to determine Radium-226 and Radium-228 in drinking waters. Radiochim Acta. https://doi.org/10.1515/ract-2018-2967
Varga ZS (2007) Preparation and characterization of manganese dioxide impregnated resin for radionuclide pre-concentration. Appl Radiat Isot 65:1095–1100. https://doi.org/10.1016/j.apradiso.2007.05.001
Kaminski MD, Mertz CJ, Ferrandon M, Dietz NL, Sandi G (2009) Physical properties of alumino – silicates waste form for cesium and strontium. J Nucl Mater 293:510–518. https://doi.org/10.1016/j.jnucmat.2009.04.020
Currie LA (1999) Detection and quantification limits: origins and historical overview. Anal Chim Acta 391:127–134
Šlesarová L (2017) Concentration of 223Ra and 90Sr in water using impregnated polyamide fibre. Comenius University in Bratislava, Faculty of Natural Sciences Department of Nuclear Chemistry. Mater thesis
Ohta T, Saito T, Saito J (2003) Collection of radium isotopes in natural waters by manganese – impregnated acrylic fiber. Radioisotopes 53(1):1–11. https://doi.org/10.1016/j.apradiso.2007.05.001
Letho J, Hou X (2011) Chemistry and analysis of radionuclides: laboratory techniques and methodology. Wiley, New York
Emami FS, Puddu V, Berry RJ, Varshney V, Patwardhan SV, Perry CC, Heinz H (2014) Prediction of specific biomolecule adsorption on silica surfaces as a function of pH and particle size. Chem Mater 26:5725–5734
Silliková V, Dulanská S, Horník M, Jakubčinová J, Mátel Ľ (2020) Impregnated fly ash sorbent for cesium-137 removal from water samples. J Radioanal Nucl Chem 324:1225–1236. https://doi.org/10.1007/s10967-020-07132-6
Koulouris G (1995) Dynamic studies on sorption characteristics of 226Ra on manganese dioxide. J Radioanal Nucl Chem 193(2):269–279. https://doi.org/10.1007/BF02039884
Franus M, Wdowin M, Bandura L, Franus W (2015) Removal of environmental pollutions using zeolites from fly ash: a review. Fresenius Environ Bull 24(3a):854–866
Moore WS, Reid DF (1973) Extraction of radium from natural waters using manganese-impregnated acrylic fibers. J Geophys Res 78(36):8880–8886. https://doi.org/10.1029/JC078i036p08880
Van ESEM, Russel BC, Ivanov P, García Miranda M, Read D, Driks C, Happel S (2017) The behaviour of 226Ra in high – volume environmental water samples on TK 100 resin. J Radioanal Nucl Chem 312:105–110. https://doi.org/10.1007/s10967-017-5203-4
Chałupnik S, Franus W, Wysocka M, Gzyl G (2013) Application of zeolites for radium removal from mine water. Environ Sci Pollut Res 20(11):7900–7906. https://doi.org/10.1007/s11356-013-1877-5
Acknowledgements
This publication was created thanks to support within the Operational Program Integrated Infrastructure for the project: Research and development in medical sciences—the way to personalized treatment of serious neurological, cardiovascular and cancer diseases 313011T431, co-financed from the resources of the European Regional Development Fund. This research was part of a dissertation theses at the department of Nuclear Chemistry of Comenius University of Bratislava in the Slovak Republic.
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Silliková, V., Dulanská, S., Jakubčinová, J. et al. Fly ash sorbent modified with KMnO4 for the separation of important radionuclides. J Radioanal Nucl Chem 332, 4335–4341 (2023). https://doi.org/10.1007/s10967-023-09128-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09128-4