Abstract
Phosphogypsum is classified into naturally occurring radioactive materials containing radionuclides such as 238U, 226Ra and 232Th, and heavy metals which are dispersed in different form and concentrations and can be dangerous for humans. Determination of the concentration of radionuclides in soils and plants near a phosphate fertilizer production unit and phosphogypsum stacks was carried out by γ- and α-spectroscopy while trace elements were determined by Neutron Activation Analysis and Atomic Absorption Spectroscopy. Transfer and enrichment factors were estimated as well as radiation risks and the results led to the conclusion that the radiation hazard in the investigated region was below than the recommended level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphogypsum (PG) is a waste by-product of the phosphate fertilizer industry. The composition of the material, generated from the wet-process between calcium phosphate ore, Ca10(PO4) F2, and sulfuric acid, is CaSO4*2H2O (96%), P2O5 (1–2%), F (1.2%), SiO2 (1%) and Al2O3 (0.2%) [1,2,3].
Τhe PG production rises globally to 300 million tons per year and most is stock-piled. Approximately 5.2 tons of phosphogypsum are produced per ton of phosphoric acid (PA) in a typical facility [4, 5]. The by-product has high volume, low toxicity and according to International Atomic Energy Agency (IAEA) is classified into N.O.R.M. (Naturally Occurring Radioactive Materials) [6]. It contains radionuclides, as well as heavy metals such as Pb, Zn, Fe, Mn, Cu, Ni, Cd and As. Most of the radionuclides of the uranium and thorium series appear in the phosphoric acid and then in the phosphate fertilizer, whereas radium (and its decay products), favors the phosphogypsum because is chemically like calcium [7,8,9]. The average uranium concentration in phosphate rocks worldwide range from 25 to 50 ppm while high concentrations up to 600 ppm have been found in local deposits. The greatest percentage (80−90%) of uranium transfers in the phosphate fertilizer [10, 11].
Circa 15% of world phosphogypsum production is recycled and applied in agriculture (amended soil), constructions (cement and concrete), mine reclamation and Rare Earth extraction [12, 13]. Moreover, the majority is disposed in large stacks, usually placed in regions close to the PA producing factories like in Florida, China, Brazil and Morocco. These stacks are exposed to weathering dispersing NPs into the environment and undergo leaching process. As dust PG is carried by air and water and thus heavy metals can be transported to adjacent surface and ground water resources [14,15,16,17,18,19,20,21,22].
More than 1.7 billion tons of PG stored in the U.S. and worldwide whereas the IAEA in order to establish standards of safety for protection of health, concluded that PG with 1 Bq g−1 or less can be reused without any meaningful restriction, and with higher concentrations can still be reused safely under certain conditions [4, 6, 22]. A significant number of publications can be found in the literature concerning mainly the radiological impact of the phosphogypsum. Up to our knowledge there is lack of information concerning the dispersion of radionuclides and trace elements after long term storage of PG in the surrounding regions and their accumulation, particularly of uranium, by plants [23,24,25,26].
The aim of this work was to determine the natural radionuclides and heavy metals dispersed from PG stacks at a phosphate fertilizer production factory, in the surrounding ecosystem, to draw an overall estimation of the hazard and radiological impact from phosphogypsum. For this reason, PG samples, soils and plants were collected and analyzed in terms of minor and trace elements, determined by Instrumental Neutron Activation Analysis (INAA) and Flame Atomic Absorption Spectroscopy (AAS), and in natural radionuclides, determined by γ- and α-spectroscopy.
Materials and methods
Study area and sampling
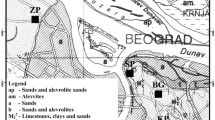
For this study 24 samples of soil and 18 of plants were collected from a region close to the phosphate fertilizer production factory, which is located at Nea Karvali in Kavala gulf in Northwestern Greece. The factory has been operating since 1965 and produces 500,000 tons phosphate fertilizer per year [27]. The samples were collected at sites 1 (next to the fence of the factory), 2 and 3 (at distance 0.5 and 1.5 km from the fence), as indicated in Fig. 1a, (corresponding coordinates, longitude and latitude A: 40° 52′ 18.1″ N 24° 30′ 01.1″ E, B: 40° 57′ 19.3″ N 24° 30′ 20.1″ E and C: 40° 57′ 27.8″ N 24° 31′ 05.4″ E). Furthermore, six PG-samples (spot 1, 2 and 3 respectively) taken from an aged PG stack (more than 15 years) were also analyzed (Fig. 1b).
Surface soil samples (circa 2 kg) were normally collected using a metallic sampler from a certain area (30 × 30 cm2) at 0–15 cm depth). Samples of native wild plants (herbs and radishes) growing around were also collected. The PG-samples were taken from the surface of the piles at different locations, according to the Environmental Protection Agency guidelines [28,29,30]. The sampling was done for one-year period, in two seasons end of March and September (wet and dry period), and in each location, three samples were collected.
Concerning the climate at the region of Kavala, is characterized by mild winters and dry, hot summers and the prevailing wind direction is south-east (average annual humidity 71% and annual rainfall 700 mm).
The samples were placed in plastic bags and transferred immediately to the laboratory. The soil samples were cleaned from stones and the plants were cut in small pieces after washing with water and distilled water. Then were dried in an oven at 60 °C until constant weight and pulverized into a fine powder after passing through a standard 1-mm mesh size (ASTM No. 18). The homogenized samples were placed into cylindrical sealed plastic beakers (volume 50 cm3) and stored for at least four weeks before the measurement to attain radioactive secular equilibrium of 226Ra, 222Rn and their short-lived progenies.
Applied techniques
For the γ-ray spectrometry a high-resolution detector was used (HPGe detector, efficiency 20%, energy resolution 2.1 keV for the 1332 keV 60Co γ-radiation). Details for the measurements have been described earlier [28]. The minor and trace elements of the samples were determined by Instrumental Neutron Activation Analysis (INAA), at the 2 MW pool-type research reactor (TU-Delft, The Netherlands, neutron dose: 4.5–5.3 × 1016 n cm−2 s−1, activation time: 4–5 h) and Flame Atomic Absorption Spectroscopy (AAS) using a Perkin Elmer AA300 spectrophotometer.
The determination of the concentrations of uranium isotopes 238U and 234U in plants was performed by alpha spectrometry after digestion and dilution of the sample using an Ortec Dual 576A alpha spectrometer and PIPS detectors (450 mm2). Prior to sample measurement, the background was carefully measured under identical conditions and was found to be about 50 counts per day within the energy range of 3–8 MeV. The efficiency of the separation using the cation exchange Chelex 100-resin was around 75% [28]. The determinations with gamma and alpha spectrometry were performed in duplicate. Radiological health indexes as Radium equivalent activity (\({\text{Ra}}_{\text{eq}}\)) in Bq kg−1, external (Hex), internal hazards (Hin), and gamma level index (Iγ) in Bq kg−1, absorbed dose rate (D) in nGy h−1, and annual effective dose equivalent, external and internal (AEDEin and AEDEex), in mSv y−1, were calculate using the following formulas 1–7 [31].
\(D({\text{nGyh}}^{ - 1} ) = 0.461\text{C}_{{{\text{Ra}}}} + 0.623 \text{C}_{{{\text{Th}}}} + 0.0414 \text{C}_{{\text{K}}}\). (5)
where CRa, CTh and CK correspond to the activities of 226Ra, 232Th and 40 K in Bq kg−1.
Contamination factor (CF) and contamination degree (Cd)
Contamination Factors (CFs) of the specific heavy metals for a specific sampling site were determined, which can be described as follows [32]
Values of CF < 1, 1 ≤ CF < 3, 3 ≤ CF < 6 and CF ≥ 6, are of evidence of low contamination, moderate contamination, considerable contamination and very high contamination.
Another index which characterizes the level of heavy metal pollution is the Contamination degree, Cd calculated from the Contamination Factors as described by Eq. (9),
where CF1, CF2, CF3, CFn represent the Contamination Factors. Values of Cd < 7, 7 ≤ Cd < 14, 14 ≤ Cd < 28 and Cd ≥ 28 indicate low contamination, moderate contamination, considerable contamination and very high contamination.
Enrichment factor (EF), transfer factor (TF)
To estimate the anthropogenic impact on soil, calculation of a normalized Enrichment Factor (EF) was performed according to the Eq. (10). This factor is referred to metal concentrations above uncontaminated background levels. The background concentrations were based on the elemental abundances of continental crust and iron (Fe) was used as the reference element for geochemical normalization because it exhibits a geochemistry like that of many trace metals and its natural mass fraction is almost uniform [33, 34].
Metal/Fe represents the mass fraction of metal to the mass fraction of Fe in the sample and in the background (reference values) respectively. When EF > 1 the element is of anthropogenic origin. Values in the range 1.5–3, 3.01–5, 5.01–10 and > 10 indicate minor, moderate, severe and very severe contamination.
Transfer Factor (TF) is defined as the ratio of the metal concentrations in plant to that in soil according to the Eq. (11) [35].
Results and discussion
The activity concentrations (Bq kg−1) of 238U, 226Ra, 232Th and 40K in PG-samples are given in Table 1 with the associated standard deviations. The gamma rays at 92.4 and 92.8 keV of 232Th were used to determine 238U based on the radioactive equilibrium. The natural radionuclides 226Ra and 232Th were determined via the decay of 214Pb (295.2 and 351.9 keV) and 214Bi (609.3, 1120.2 keV) as well as of 228Ac (911.2 and 968.9 keV), 212Pb (238.6 and 300.0 keV) and 208Tl (583.1 keV) respectively. It is shown that the sample in spot-1 is raw phosphogypsum with high activity of 226Ra and low of 232Th while in spot-2 the samples are mixed with soil and in spot-3 the constitution of the samples tends towards that of the soil. Although the activity concentrations for PG-samples, which appeared in the literature, varied significantly (for example for 226Ra different values have been reported; 310 Bq kg−1 in Iraq and Syria, 462 Bq kg−1 in Greece, 591/811 Bq kg−1 in Brazil, 958 Bq kg−1 in Florida and 1420 Bq kg−1 in Morocco), the determined values in this study were in the worldwide range [22, 25, 26].
Radiological indexes such as \({\text{Ra}}_{\text{eq}}\), D, Iγ, Hex and Hin, are given in Table 2. The \({\text{Ra}}_{\text{eq}}\) value in spot-1 was higher than the recommended accepted limit of 370 Bq kg−1, for safe use [31]. The Iγ values for the spots 1, 2 and 3 reflecting the dispersion of phosphogypsum and ranged between 0.83 and 3.11 Bq kg−1 while \(\text{the}\) annual effective dose ranged between 0.48 and 1.42 mSv y−1 and was always higher than the recommended value 0.46 mSv y−1.
The elemental analysis of the PG samples identified by INAA is presented in Table 3 where can be seen that some metals, such as rare earths, arsenic, zinc and uranium, are present in smaller concentrations than the average values in literature [19]. As it is known the constitution of phosphogypsum depends on the origin of the phosphate rock and the age of the PG-piles. A significant amount (~ 85%) of the rare earths elements (REE) and mobile metals as uranium and zinc passes in the PG matrix but no significant enrichment of REE has been observed in aged PG-piles [21, 36, 37]. Generally, both concentrations of metals and concentrations of trace elements in PG samples, in this work agree with literature values [20, 23].
The activity concentrations of the 238U, 226Ra and 232Th radionuclides for the soil samples, 9 including minimum, maximum, mean values and standard deviations are presented in Table 4. The values ranged from 16.8 to 38.9, 27.3 to 44.4 and 31.2 to 83.8 Bq kg−1 for 238U, 226Ra and 232Th. The differences between cold and warm periods were negligible. Uranium and radium seem to follow the same trend with higher activity concentrations in spring in two sampling sites as it is shown in Fig. 2. The opposite was observed in site 3 likely as consequence of the wind direction in the region. For uranium the difference between the two seasons was higher, probably because this element is transferred from the phosphate rock to the bioavailable fraction in phosphogypsum and finally in the water-soluble phase, being dangerous for the environment [38]. The mobility of the metals is a complex matter depending, among others (pH, speciation, etc.) and on weather conditions. The regional climate, as has been also mentioned, is of a Mediterranean type and characterized by rainy winters and warm-dry summers. This means that in rainy periods, elements bounded to the bioavailable fraction, form soluble phases in rainwater, and released into the surrounding environment [8, 9, 36,37,38,39].
The recorded values of the measured radionuclides in soils are in the range of the worldwide activity concentrations but higher than the world mean values of 238U (35 Bq kg−1), 226Ra (35 Bq kg−1), 232Th (30 (Bq kg−1) and 40K (400 Bq kg−1), recommended by UNSCEAR 2000 [9, 22, 26, 28, 36,37,38,39]. Similar observations have been reported by other researchers in relevant studies in Nigeria, Greece, Tunisia, and Syria [15, 22, 37,38,39]. The radiological indices are tabulated in Table 5 and illustrated in Fig. 3. The \({\text{Ra}}_{\text{eq}}\) activity varied from 113.3 to 194.2 Bq kg−1 lower than the recommended value. The values of external (Hex) and internal hazards (Hin) and Gamma level index (Iγ) were always lower than unity indicating that the soil in this area is safe for use.
The concentrations of heavy metals in the investigated regions are given in Table 6 together with literature data for comparison whereas their distribution is presented in Fig. 4 (except of Zn which had concentrations from 161.2 up to 238.7 mg kg−1). As it is shown the accumulation of metals in this region is lower in comparison to other reported data [40,41,42,43,44,45]. Concerning the metal spatial dispersion, no safe conclusion can be drawn because metals due to their high density can be dispersed as dust by wind and deposited near the surrounding area [37]. Moreover, the incidental NPs from the PG stacks contribute to the distribution of REE, toxic elements such as As, Pb and Cd, and natural radionuclides [19].
The average value of the Contamination Factor (CF) was lower than unity for the elements As, Pb, Cu and Ni and between 1 and 3 for Zn, Cd, U and Th, signifying low and moderate contamination respectively which was also proven and by the Contamination degree (Cd). Figure 5 illustrates the Enrichment Factor (EF) of the metals in the soils close to the fertilizer unit where slight enrichment was observed for most of the metals and moderate enrichment for Zn.
The metal concentrations in plants are presented in Table 7 along with published data for comparison. Most of these data refer to different pollution sources because there is a lack of knowledge about the impact of phosphogypsum on plants grown naturally in the vicinity of PG-stockpiles [8, 23, 37]. As it is shown in most of the cases the values in this work were lower than relevant literature data reflecting the moderate contamination in the region. Slight differences were observed in different points and in different season as can be seen in Fig. 6 with higher values in rainy period (spring) [37,38,39,40,41,42,43,44,45,46]. The transfer of heavy metals to plants is not only due to the absorption by the plants’ roots but also due to the precipitation on the leaves by rain or wind which could not be removed by washing. Generally highest TF values were observed in the literature for the leafy plants while lowest values were observed for grains and the accumulation of metals in plants is conected with the soil pollution [8, 23, 37, 42].
The determined concentrations for uranium ranged between 0.3 and 0.7 (mg kg−1) but general the results referred to uranium in plants are very limited. In Russia they found concentrations up to 20 mg kg−1 for native plants. Uranium concentrations between 0.15 and 0.25 and 1–3 mg kg−1 were reported for vegetables in Italy and Vietnam [46,47,48,49,50]. It is worth to be mentioned that usually, the plants investigated in the literature concern vegetables and not native wild plants.
Figure 7 presents the Transfer Factor (TF) of the metals from soil to plants. The values found in this study are according to these reported by other researchers (e.g. Mazzilli et al. in Brazil) and lead to the conclusion that the transfer of toxic metals from the fertilizer unit to the surrounding environment is limited [23]. Of course, there is a large variability among TF data due to many parameters concerning soil, plants group and crop. In this study uranium and cooper exhibited the highest TF values, followed the metals Zn, Ni and Pb, while low TF values were exhibited by Cd and As [8, 9].
Conclusions
To assess the hazard from radiological and chemical pollution due to dispersion of radionuclides and heavy metals from phosphogypsum stacks in the surrounding region, soil and native wild plants were investigated. For the soil samples the activities of the radionuclides were comparable to the literature and the values of external (Hex), internal hazards (Hin) and Gamma level index (Iγ) were always lower than unity indicating safe use of the soil. The metal concentrations were in agreement with results appeared in the literature and the Contamination Factors and Contamination degree signifying low and moderate contamination. Slight enrichment of the soils was observed for most of the metals and moderate enrichment for Zn according to Enrichment Factor values.
For the plants in most of the cases the metal concentrations were lower than relevant literature data reflecting the moderate contamination in the region. Slight differences were observed in different points and in different season with higher values in rainy period (spring) due to the precipitation by rain. The TF values showed variability due to many parameters concerning soil, plants group and crop.
References
Saadaoui E, Ghazel N, Romdhane CB, Massoudi N (2017) Phosphogypsum: potential uses and problems—a review. Int J Environ Stud 74(4):558–567
Borges RC, Ribeiro FCA, Da Costa LD, Bernedo AVB (2013) Radioactive characterization of phosphogypsum from Imbituba, Brazil. J Environ Radioact 126:188–195
Gezer F, Turhan S, Ugur FA, Goren E, Kurt MZ, Ufuktepe Y (2012) Natural radionuclide content of disposed phosphogypsum as TENORM produced from phοsphorus fertilizer industry in Turkey. Ann Nucl Energy 50:33–37
Layr K, Hartlieb P (2019) Market analysis for urban mining of phosphogypsum. BHM Bergund Hüttenmännische Monatshefte 164:245–249
Yang L, Zhang Y, Yan Y (2016) Utilization of original phosphogypsum as raw material for the preparation of self-leveling mortar. J Clean Prod 127:204–213
https://www-pub.iaea.org General Safety Guide No. GSG-8 (assessed 2 December 2022)
Haridasan PP, Maniyan CG, Pillai PMB, Khan AH (2002) Dissolution characteristics of 226Ra from phosphogypsum. J Environ Radioact 62:287–294
Al-Hwaiti MS, Ranville JF, Ross PE (2010) Bioavailability and mobility of trace metals in phosphogypsum from Aqaba and Eshidiya, Jordan. Chem Erde 70:283–291
Jalali J, Gaudin P, Capiaux H, Ammar E, Lebeau T (2019) Fate and transport of metal trace elements from phosphogypsum piles in Tunisia and their impact on soil bacteria and wild plants. Ecotoxicol Environ Saf 174:12–25
Haneklaus N, Sun Y, Bol R, Lottermoser B, Schnug E (2017) To Extract, or not to extract uranium from phosphate rock, that is the question. Environ Sci Technol 51:753–754
Bilal E et al (2023) Phosphogypsum circular economy considerations: a critical review from more than 65 storage sites worldwide. J Clean Prod 414:137561
Papastefanou C, Stoulos S, Ioannidou A, Manolopoulou M (2006) The application of phosphogypsum in agriculture and the radiological impact. J Environ Radioact 89:188–198
Pantazopoulou E, Zebiliadou O, Noli F, Mitrakas M, Samaras P, Zouboulis A (2015) Utilization of phosphogypsum in tannery sludge stabilization and evaluation of the radiological impact. Bull Environ Contam Toxicol 94:352–357
Burnett WC, Elzerman AW (2001) Nuclide migration and the environmental radiochemistry of Florida phosphogypsum. J Environ Radioact 54:27–51
Okeji MC, Agwu KK, Idigo FU (2012) Assessment of natural radioactivity in phosphate ore, phosphogypsum, and soil samples around a phosphate fertilizer plant in Nigeria. Bull Environ Contam Toxicol 89:1078–1081
Al-Masri MS, Amin Y, Ibrahim S, Al-Bich F (2004) Distribution of some trace metals in Syrian phosphogypsum. Appl Geochem 19:747–753
Azouazi M, Ouahidi Y, Fakhi S, Andres Y, Abbe JC, Benmansour M (2001) Natural radioactivity in phosphates, phosphogypsum and natural waters in Morocco. J Environ Radioact 54:231–242
Potiriadis C, Koukouliou V, Seferlis S, Kehagia K (2011) Assessment of the occupational exposure at a fertilizer industry in the northern part of Greece. Radiat Prot Dosim 144(1–4):668–671
Silva NC, Fernandes EAN, Cipriani M, Taddei MHT (2001) The natural radioactivity of Brazilian phosphogypsum. J Radioanal Nucl Chem 249(1):251–255
Villalobos MR, Vioque I, Mantero J, Manjon G (2010) Radiological, chemical and morphological characterizations of phosphate rock and phosphogypsum from phosphoric acid factories in SW Spain. J Hazard Mater 181:193–203
Zielinski RA, Al-Hwaiti MS, Budahn JR, Ranville JF (2011) Radionuclides, trace elements, and radium residence in phosphogypsum of Jordan. Environ Geochem Health 33:149–165
Papageorgiou F, Godelitsas A, Mertzimekis TJ, Xanthos S, Voulgaris N, Katsantonis G (2016) Environmental impact of phosphogypsum stockpilein remediated Schistos waste site (Piraeus, Greece) using a combination of γ-ray spectrometry with geographic information systems. Environ Monit Assess 188(133):1–14
Mazzilli BP, Saueia CHR, Jacomino VMF (2012) Natural radionuclides and metals intake into soya, corn and lettuce grown on soil amended with phosphogypsum. Int J Environ Anal Chem 92(14):1574–1586
Contreras M, Pérez-López R, Gázque MJ, Morales-Flórez V, Santos A, Esquivias L, Bolívar JP (2015) Fractionation and fluxes of metals and radionuclides during the recycling process of phosphogypsum wastes applied to mineral CO2 sequestration. J Waste Manag 45:412–419
Ndour O, Thiandoume C, Traore A, Cagnat X, Diouf P, Mbaye N, Maurice N, Ababacar S, Tidjani A (2021) Determination of natural radionuclides in phosphogypsum samples from phosphoric acid production industry in Senegal. Environ Forensics 1–8
Jia G, Buchetti M, Conti D, Magro L, Mariani S (2022) Radioecological studies of the main naturally occurring radionuclides in the area of Gela Phosphate Industry (Italy) through radioanalytical separation and measurement techniques. Appl Radiat Isot 184:110173
Noli F, Tsamos P (2018) Seasonal variations of natural radionuclides, minor and trace elements in lake sediments and water in a lignite mining area of North-Western Greece. Environ Sci Pollut Res 25:12222–12233
Tsamos P, Stoulos S, Noli F (2022) Radiological status in sea-sediments and sand near an oil shale power plant in North-Western Greece. J Radioanal Nucl Chem 331(9):3703–3711
USEPA (2002) national emission standards for hazardous air pollutants, Subpart R
Bangotra P, Mehra R, Jakhu R, Kaur K, Pandit P, Kanse S (2018) Estimation of 222Rn exhalation rate and assessment of radiological risk from activity concentration of 226Ra, 232Th and 40K. J Geochem Explor 184:304–310
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001
Jolly Y, Islam A, Akbar S (2013) Transfer of metals from soil to vegetables and possible health risk assessment. Springer Plus 2(385):1–8
Tamim U, Khan R, Jolly YN, Fatema K, Das S, Naher K, Islam MA, Azharul Islam SM, Hossain SM (2016) Elemental distribution of metals in urban river sediments near an industrial effluent source. Chemosphere 155:509–518
Rudnick RL, Gao S (2014) Composition of the continental crust. Treatise Geochem 3:1–51
Zmemla R, Sdiri A, Naifar I, Benjdidia M, Elleuch B (2020) Tunisian phosphogypsum tailings: assessment of leaching behavior for an integrated management approach. Environ Eng Res 25(3):345–355
Al-Attar L, Al-Oudat M, Kanakri S, Budeir Y, Khalily H, Al-Hamwi A (2011) J Environ Manag 92:2151–2158
Perez-Lopez R, Lvarez-Valero AMA, Nieto JM (2007) Changes in mobility of toxic elements during the production of phosphoric acid in the fertilizer industry of Huelva (SW Spain) and environmental impact of phosphogypsum wastes. J Hazard Mater 148:745–750
UNSCEAR (2000) Sources and effects of ionizing radiation. United Nations, New York
Noli F, Tsamos P, Stoulos S (2017) Spatial and seasonal variation of radionuclides in soils and waters near a coal-fired power plant of Northern Greece: environmental dose assessment. J Radioanal Nucl Chem 311:331–338
Noli F, Tsamos P (2016) Concentrations of heavy metals and trace elements in soils, waters and vegetables and health risk in the vicinity of a lignite-fired power plant. Sci Total Environ 563–564:377–385
Alina-Pendias K (2011) Trace elements in soils and plants. CRC Press, Cambridge
Kicińska A, Wikar J (2021) The effect of fertilizing soils degraded by the metallurgical industry on the content of elements in Lactuca sativa L. Sci Rep 11(4072):1–17
Folens K, Van Labeke MC, Du Laing G (2017) Impact of an urban environment on trace element concentrations in domestically produced lettuce (Lactuca sativa L.). Water Air Soil Pollut 228(457):1–12
Voutsa D, Samara C (1998) Dietary intake of trace elements and polycyclic aromatic hydrocarbons via vegetables grown in an industrial Greek area. Sci Total Environ 218:203–216
Huang L, Liu Y, Ferreira J, Wang M, Na J, Huang J, Liang Z (2022) Long-term combined effects of tillage and rice cultivation with phosphogypsum or farmyard manure on the concentration of salts, minerals, and heavy metals of saline-sodic paddy fields in Northeast China. Soil Till Res 215:105222
Shtangeeva I (2010) Uptake of uranium and thorium by native and cultivated plants. J Environ Radioact 101:458–463
Nali C, Balducci E, Frati L, Paoli L, Loppi S, Lorenzini G (2009) Lettuce plants as bioaccumulators of trace elements in a community of central Italy. Environ Monit Assess 149:143–149
Thien BN, Ba VN, Thao NTV, Loan TTH (2020) Estimation of the soil to plant transfer factor and the annual organ equivalent dose due to ingestion of food crops in Ho Chi Minh city, Vietnam. Chemosphere 259:127432
Vandenhove H, Olyslaegers G, Sanzharova N, Shubina O, Reed E, Shang Z, Velasco H (2009) Proposal for new best estimates of the soil-to-plant transfer factor of U, Th, Ra, Pb and Po. J Environ Radioact 100:721–732
Acknowledgements
The authors would like to thank the group of INAA at TU-Delft, The Netherlands for help with the measurements (Programme, NMI3-II Grant No. 283883).
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Noli, F., Sidirelli, M. & Tsamos, P. Dispersion of radionuclides and heavy metals from phosphogypsum stacks in soil and plants at Northwestern Greece. J Radioanal Nucl Chem 332, 4213–4221 (2023). https://doi.org/10.1007/s10967-023-09120-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09120-y