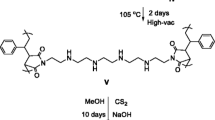
Abstract
A new poly(4-vinylpyridine) resin was prepared and characterized. The adsorption of Th4+ on resin was studied as a function of contact time, nitric acid concentration, metal ion impurity and initial Th4+ concentration. The adsorption kinetics and isotherms were analyzed. The results revealed that the adsorption equilibrium for the adsorption of Th4+ on resin was reached within 30 min. The efficiency of Th4+ adsorption on resin improves when the nitric acid acidity increases. The adsorption capacity of the resin increases with an increase in the initial concentration of Th4+. The thorium distribution coefficient increases with metal nitrate in the system.










Similar content being viewed by others
References
Pribyl JG, Taylor-Pashow KML, Shehee TC, Benicewicz BC (2018) High-capacity poly(4-vinylpyridine) grafted PolyHIPE foams for efficient plutonium separation and purification. ACS Omega 3:8181–8189. https://doi.org/10.1021/acsomega.8b01057
Ryan JL, Wheelwright EJ (1959) Recovery and purification of plutonium by anion exchange. Ind Eng Chem 51:60–65. https://doi.org/10.1021/ie50589a038
Barr ME, Jarvinen GD, Moody EW, Vaughn R, Silks LA, Bartsch RA (2007) Plutonium(IV) sorption by soluble anion-exchange polymers. Sep Sci Technol 37:1065–1078. https://doi.org/10.1081/SS-120002241
James D, Navratil YW (2001) Actinide ion exchange technology in the back end of the nuclear fuel cycle. Nukleonika 46:75–80
Nogami M, Fujii Y, Sugo T (1996) Radiation resistance of pyridine type anion exchange resins for spent full treatment. J Radioanal Nucl Chem 203:109–117. https://doi.org/10.1007/BF02060385
Marsh SF (1992) The comparative effects of gamma radiation and in situ alpha particles on five strong-base anion exchange resins. https://doi.org/10.1007/978-94-011-2864-3_47
Pillay K (1986) A review of the radiation stability of ion exchange materials. J Radioanal Nucl Chem 102:247–268. https://doi.org/10.1007/BF02037966
Walter WJCEAKSR (2000) Qualification of ReillexTM HPQ anion exchange resin for use in SRS processes. https://doi.org/10.2172/755372
Kyser EA (2001) Plutonium loading onto Reillex HPQ anion exchange resin. https://doi.org/10.2172/773118
Marsh S, Veirs D, Jarvinen G (2000) Molecularly engineered resins for plutonium recovery. Los Aalmos Science 26:454–463
Korkisch J, Arrhenius G (1964) Separation of uranium, thorium, and the rare earth elements by anion exchange. Anal Chem 36:850–854. https://doi.org/10.1021/ac60210a044
Arai T, Wei Y, Kumagai M, Horiguchi K (2006) Separation of rare earths in nitric acid medium by a novel silica-based pyridinium anion exchange resin. J Alloys Compd 412:1008–1012. https://doi.org/10.1016/j.jallcom.2004.11.092
Xiong XH, Yuan YH, Huang B, He M, Chen H, Luo YC, Zhu YA, Luo TA, Chen QS (2018) Th(IV) adsorption onto titanium tetrachloride modified sodium bentonite. J Radioanal Nucl Chem 319:805–815
Xuebin W, Guoshu M, Zhihai F, Jin Z, Xian Z, Shengjie Y, Ruochen Z (2022) Study on adsorption properties of am in solution containing impurities by TODGA resin. wwwgdchemcom 49:45–48
Zhi-hui D, Ming-chun J, Jin-feng M, Shi-bin FENG (2014) Preparation of polyacrylonitrile-potassium cobalt/titanium hexacyanoferrate(II)spherical composite adsorbents and their adsorption properties for Cs+. Atomic Energy Sci Technol 48:14–22. https://doi.org/10.7538/yzk.2014.48.01.0014
Liu D, Liu Z, Wang C, Lai Y (2016) Removal of uranium(VI) from aqueous solution using nanoscale zero-valent iron supported on activated charcoal. J Radioanal Nucl Chem 310:1131–1137. https://doi.org/10.1007/s10967-016-4892-4
Altinisik A, Gur E, Seki Y (2010) A natural sorbent, Luffa cylindrica for the removal of a model basic dye. J Hazard Mater 179:658–664. https://doi.org/10.1016/j.jhazmat.2010.03.053
Chen YL, Zhao L, Wei YZ, He LF, Tang FD (2015) Adsorption characteristics of thorium on silica-based anion exchange resins. Nucl Sci Tech 26:1–8. https://doi.org/10.13538/j.1001-8042/nst.26.060303
Hosseini MS, Hosseini-Bandegharaei A (2009) Selective extraction of Th(IV) over U(VI) and other co-existing ions using eosin B-impregnated Amberlite IRA-410 resin beads. J Radioanal Nucl Chem 283:23–30. https://doi.org/10.1007/s10967-009-0037-3
Pathak SK, Tripathi SC, Singh KK, Mahtele AK, Kumar M, Gandhi PM (2015) Simultaneous separation and purification of plutonium and americium from aqueous nitrate solutions using extractant impregnated macroporous polymeric beads. J Radioanal Nucl Chem 308:47–57. https://doi.org/10.1007/s10967-015-4330-z
Sun X, Huang X, Liao XP, Shi B (2011) Adsorptive removal of Cu(II) from aqueous solutions using collagen-tannin resin. J Hazard Mater 186:1058–1063. https://doi.org/10.1016/j.jhazmat.2010.11.098
Fredric Marsh S (1989) Reillex HPQ: a new, macroporous polyvinylpyridine resin for separating plutonium using nitrate anion exchange. Solvent Extr Ion Exch 7:889–908. https://doi.org/10.1080/07360298908962344
Funding
No Funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, R., Mao, G., Fu, Z. et al. Adsorption of Th(IV) in nitric acid medium by a new poly(4-vinylpyridine) resin. J Radioanal Nucl Chem 332, 3903–3910 (2023). https://doi.org/10.1007/s10967-023-09072-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09072-3