Abstract
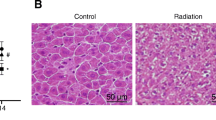
This study aimed to investigate the efficacy of ethyl cinnamate (EC) (75 μg/ml) in ameliorating the effects of γ-radiation (2, 5, and 8 Gy) on normal liver BRL-3A cells at 1 and 24 h post-irradiation. γ-radiation-induced persistent damage to normal hepatocytes is partly mediated by reactive oxygen species (ROS)-regulated early apoptosis and prolonged inhibition of survival factors. EC treatment increased superoxide dismutase (SOD) activity, decreased ROS yield and lipid peroxidation (LPO), activated the PI3-AKT pathway and p-Bcl-2, and reduced cleaved caspase -3, - 9, and Bax. This study highlights for the first time the potential of ethyl cinnamate as an efficient radioprotector.







Similar content being viewed by others
References
Kim J, Jung Y (2017) Radiation-induced liver disease: current understanding and future perspectives. Exp Mol Med 49(7):e359
Toesca DAS, Ibragimov B, Koong AJ, Xing L, Koong AC, Chang DT (2018) Strategies for prediction and mitigation of radiation-induced liver toxicity. J Radiat Res 59:40–49
Raza A, Sood GK (2014) Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol 20(15):4115–4127
Zhang T, Merle P, Wang H, Zhao H, Kudo M (2021) Combination therapy for advanced hepatocellular carcinoma: do we see the light at the end of the tunnel? Hepatobiliary Surg Nutr 10(2):180–192
Ohri N, Dawson LA, Krishnan S, Seong J, Cheng JC, Sarin SK, Kinkhabwala M, Ahmed MM, Vikram B, Coleman CN, Guha C (2016) Radiotherapy for hepatocellular carcinoma: new indications and directions for future study. J Natl Cancer Inst 108(9):djw133
Zhu W, Zhang X, Yu M, Lin B, Yu C (2021) Radiation-induced liver injury and hepatocyte senescence. Cell Death Discov 7:244
Liu R, Bian Y, Liu L, Liu L, Liu X, Ma S (2022) Molecular pathways associated with oxidative stress and their potential applications in radiotherapy (Review). Int J Mol Med 49(5):65
Blanco Y, de Diego-Castilla G, Viúdez-Moreiras D, Cavalcante-Silva E, Rodríguez-Manfredi JA, Davila AF, McKay CP, Parro V (2018) Effects of gamma and electron radiation on the structural integrity of organic molecules and macromolecular biomarkers measured by microarray immunoassays and their astrobiological implications. Astrobiology 18(12):1497–1516
Na K, Cho Y, Choi D-H, Park M-J, Yang J-H, Chung S (2021) Gamma irradiation exposure for collapsed cell junctions and reduced angiogenesis of 3-D in vitro blood vessels. Sci Rep 11:18230
Maia GA, de Oliveira RC, Medina JM, da Silveira AB, Mignaco JA, Atella GC, Cortes VF, Barbosa LA, de Lima SH (2014) The effect of gamma radiation on the lipid profile of irradiated red blood cells. Ann Hematol 93:753–760
Plichta K, Camden N, Furqan M, AbuHejleh T, Clamon GH, Zhang J, Flynn RT, Bhatia SK, Smith MC, Buatti JM, Allen BG (2017) SBRT to adrenal metastases provides high local control with minimal toxicity. Adv Radiat Oncol 2(4):581–587
Koay EJ, Owen D, Das P (2018) Radiation-induced liver disease and modern radiotherapy. Semin Radiat Oncol 28(4):321–331
Benson R, Madan R, Kilambi R, Chander S (2016) Radiation induced liver disease: a clinical update. J Egypt Natl Cancer Inst 28(1):7–11
Abdollahi H, Shiri I, Atashzar M, Sarebani M, Moloudi K, Samadian H (2015) Radiation protection and secondary cancer prevention using biological radioprotectors in radiotherapy. Int J Cancer Ther Oncol 3(3):335
Nemoto K, Horiuchi K, Miyamoto T (1995) Deoxyspergualin is a new radioprotector in mice. Radiat Res 141(2):223–226
Kamran MZ, Ranjan A, Kaur N, Sur S, Tandon V (2016) Radioprotective agents: strategies and translational advances. Med Res Rev 36:461–493
Weiss JF, Kumar KS, Walden TL, Neta R, Landauer MR, Clark EP (1990) Advances in radioprotection through the use of combined agent regimens. Int J Rad Biol 57(4):709–722
Checker R, Patwardhan RS, Jayakumar S, Maurya DK, Bandekar M, Sharma D, Sandur SK (2021) Chemical and biological basis for development of novel radioprotective drugs for cancer therapy. Free Radic Res 55(8):828–858
Kamran MZ, Ranjan A, Kaur N, Sur S, Tandon V (2016) Radioprotective agents: strategies and translational advances. Med Res Rev 36(3):461–493
Hosseinimehr SJ (2007) Trends in the development of radioprotective agents. Drug Discov Today 12(19–20):794–805
da Costa Araldi IC, Bordin FPR, Cadoná FC, Barbisan F, Azzolin VF, Teixeira CF, Baumhardt T, da Cruz IBM, Duarte MMMF, Bauermann LF (2018) The in vitro radiosensitizer potential of resveratrol on MCF-7 breast cancer cells. Chem-Biol Interact 282:85–92
Tan Y, Wei X, Zhang W, Wang X, Wang K, Du B, Xiao J (2017) Resveratrol enhances the radiosensitivity of nasopharyngeal carcinoma cells by downregulating E2F1. Oncol Rep 37(3):1833–1841
Baek SH, Ko JH, Lee H, Jung J, Kong M, Lee JW, Lee J, Chinnathambi A, Zayed ME, Alharbi SA et al (2016) Resveratrol inhibits STAT3 signalling pathway through the induction of SOCS-1: Role in apoptosis induction and radiosensitisation in head and neck tumor cells. Phytomedicine 23(5):566–577
Minafra L, Porcino N, Bravatà V, Gaglio D, Bonanomi M, Amore E, Cammarata FP, Russo G, Militello C, Savoca G et al (2019) Radiosensitizing effect of curcumin-loaded lipid nanoparticles in breast cancer cells. Sci Rep 9:11134
Schwarz K, Dobiasch S, Nguyen L, Schilling D, Combs SE (2020) Modification of radiosensitivity by Curcumin in human pancreatic cancer cell lines. Sci Rep 10:3815
Karthikeyan S, Kanimozhi G, Prasad NR, Mahalakshmi R (2011) Radiosensitizing effect of ferulic acid on human cervical carcinoma cells in vitro. Toxicol In Vitro 25(7):1366–1375
Singh Tuli H, Kumar A, Ramniwas S, Coudhary R, Aggarwal D, Kumar M, Sharma U, Chaturvedi Parashar N, Haque S, Sak K (2022) Ferulic acid: a natural phenol that inhibits neoplastic events through modulation of oncogenic signaling. Molecules 27(21):7653
Sebastia N, Montoro A, Hervas D, Pantelias G, Hatzi VI, Soriano JM, Villaescusa JI, Terzoudi GI (2014) Curcumin and trans-resveratrol exert cell cycle-dependent radioprotective or radiosensitising effects as elucidated by the PCC and G2-assay. Mutat Res 766–767:49–55
Jiao Y, Ouyang HL, Jiang YJ, Kong XZ, He W, Liu WX, Yang B, Xu FL (2015) Toxic effects of ethyl cinnamate on the photosynthesis and physiological characteristics of Chlorella vulgaris based on chlorophyll fluorescence and flow cytometry analysis. Sci World J 2015:107823
Gao LL, Guo PY, Su GM, Wei YF (2013) Effects of allelochemicals ethyl cinnamate on the growth and physiological characteristics of Chlorella pyrenoidosa. Huan Jing Ke Xue 34(1):156–162
Zhang B, Lv C, Li W, Cui Z, Chen D, Cao F, Miao F, Zhou L (2015) Ethyl cinnamate derivatives as promising high-efficient acaricides against Psoroptes cuniculi: synthesis, bioactivity and structure-activity relationship. Chem Pharm Bull 63(4):255–262
Dubey NK, Tiwari TN, Mandin D, Andriamboavonjy H, Chaumont JP (2000) Antifungal properties of Ocimum gratissimum essential oil (ethyl cinnamate chemotype). Fitoterapia 71(5):567–569
Mukherjee S, Dutta A, Chakraborty A (2022) The cross-talk between Bax, Bcl2, caspases, and DNA damage in bystander HepG2 cells is regulated by γ-radiation dose and time of conditioned media transfer. Apoptosis 27(3–4):184–205
Ma ZC, Hong Q, Wang YG, Tan HL, Xiao CR, Liang QD, Wang DG, Gao Y (2011) Ferulic acid protects lymphocytes from radiation-predisposed oxidative stress through extracellular regulated kinase. Int J Radiat Biol 87(2):130–140
Mishra K, Ojha H, Kallepalli S, Alok A, Chaudhury NK (2014) Protective effect of ferulic acid on ionising radiation induced damage in bovine serum albumin. Int J Rad Res 12(2):113–121
Maurya DK, Devasagayam TPA (2013) Ferulic acid inhibits gamma radiation-induced DNA strand breaks and enhances the survival of mice. Cancer Biother Radiopharm 28(1):51–57
Shin HA, Shin YS, Kang SU, Kim JH, Oh YT, Park KH, Lee BH, Kim CH (2014) Radioprotective effect of epicatechin in cultured human fibroblasts and zebrafish. J Rad Res 55(1):32–40
Das DK, Sinha M, Khan A, Das K, Manna K, Dey S (2013) Radiation protection by major tea polyphenol. Epicatechin Int J Hum Genet 13(1):59–64
Sinha M, Das DK, Manna K, Datta S, Ray T, Sil AK, Dey S (2012) Epicatechin ameliorates ionizing radiation-induced oxidative stress in mouse liver. Free Rad Res 46(7):842–849
Morgan WF (2003) Is there a common mechanism underlying genomic instability, bystander effects and other nontargeted effects of exposure to ionizing radiation? Oncogene 22(45):7094–7099
Robbins ME, Zhao W (2004) Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol 80(4):251–259
Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD (2004) Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci 117(Pt 11):2417–2426
Dong S, Lyu X, Yuan S, Wang S, Li W, Chen Z, Yu H, Li F, Jiang Q (2020) Oxidative stress: a critical hint in ionizing radiation induced pyroptosis. Radiat Med Prot 1(4):179–185
Datta K, Suman S, Kallakury BV, Fornace AJ Jr (2012) Exposure to heavy ion radiation induces persistent oxidative stress in mouse intestine. PLoS ONE 7(8):e42224
Shojaie L, Iorga A, Dara L (2020) Cell death in liver diseases: a review. Int J Mol Sci 21(24):9682
Fukuda K, Uehara Y, Nakata E, Inoue M, Shimazu K, Yoshida T, Kanda H, Nanjo H, Hosoi Y, Yamakoshi H et al (2016) A diarylpentanoid curcumin analog exhibits improved radioprotective potential in the intestinal mucosa. Int J Rad Biol 92(7):388–394
Kumar S, Tiku AB (2016) Biochemical and molecular mechanisms of Radioprotective effects of Naringenin, a phytochemical from citrus fruits. Agric Food Chem 64(8):1676–1685
Das U, Biswas S, Sengupta A, Manna K, Chakraborty A, Dey S (2016) Ferulic acid (FA) abrogates ionising radiation-induced oxidative damage in murine spleen. Int J Rad Biol 92(12):806–818
Sam F, Kerstetter DL, Pimental DR, Mulukutla S, Tabaee A, Bristow MR, Colucci WS, Sawyer DB (2005) Increased reactive oxygen species production and functional alterations in antioxidant enzymes in human failing myocardium. J Card Fail 11(6):473–480
Yano S, Yano N (2002) Regulation of catalase enzyme activity by cell signaling molecules. Mol Cell Biochem 240(1–2):119–130
Kim GJ, Fiskum GM, Morgan WF (2006) A role for mitochondrial dysfunction in perpetuating radiation-induced genomic instability. Cancer Res 66(21):10377–10383
Ekaterina P, Ekaterina L, Eugenia D, Nadezhda Z, Anna K, Mikhail S, Alexey M (2016) Geroprotective Radioprotective activity of quercetin, (-)-Epicatechin, and ibuprofen in drosophila melanogaster. Front Pharmacol 7:505
Sruthi P, Naidu MM (2021) Radioprotection by curcumin: a mini review. J Spices Aromat Crops 30(1):24–33
Son Y, Kim S, Chung HT, Pae HO (2013) Reactive oxygen species in the activation of MAP kinases. Methods Enzymol 528:27–48
Koundouros N, Poulogiannis G (2018) Phosphoinositide 3-kinase/Akt signaling and redox metabolism in cancer. Front Oncol 8:160
Xie W, Huang W, Cai S, Chen H, Fu W, Chen Z, Liu Y (2021) NF κB/IκBα signaling pathways are essential for resistance to heat stress induced ROS production in pulmonary microvascular endothelial cells. Mol Med Rep 24(5):814
Hickling KC, Hitchcock JM, Oreffo V, Mally A, Hammond TG, Evans JG, Chipman JK (2010) Evidence of oxidative stress and associated DNA damage, increased proliferative drive, and altered gene expression in rat liver produced by the cholangiocarcinogenic agent furan. Toxicol Pathol 38(2):230–243
Acknowledgements
The author AD thankfully acknowledges the University Grant Commission (UGC), Government of India, for funding the project (Sanction no. F.15-6 Dec 2014/2015 NET). The author SM would like to thank the Indian Council of Medical Research (ICMR) for providing a research grant (No. 3/1/3/JRF-2016/HRD). The authors thank the UGC-DAE Consortium for Scientific Research, Kolkata Centre, India, for providing all the facilities for conducting this research.
Author information
Authors and Affiliations
Contributions
Conceptualization: SM, AD, AC; Methodology: SM, AD; Formal analysis and investigation: SM, AD; Writing—original draft preparation: SM, AD; Writing—review and editing: SM, AD, AC; Funding acquisition: SM, AD; Supervision: AC All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mukherjee, S., Dutta, A. & Chakraborty, A. γ-radiation-induced damage on normal hepatocytes and its protection by ethyl cinnamate. J Radioanal Nucl Chem 333, 1453–1465 (2024). https://doi.org/10.1007/s10967-023-09067-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09067-0