Abstract
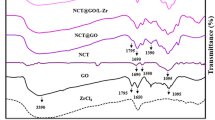
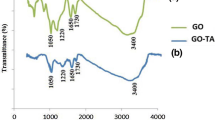
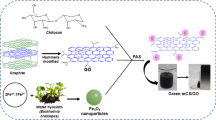
A new persimmon tannin-modified graphene oxide/chitosan microsphere (PGC) was prepared by the mixed crosslinking method, and the effective adsorption behavior of U(VI) in an aqueous solution was discussed. The study shows that at the temperature of 30 °C, pH of 5, time of 120 min, and the an initial uranium concentration of 10.00 mg/L, the maximum adsorption capacity of PGC microspheres for U(VI) reached 199.13 mg/g, with a removal rate of 98.2%. The adsorption process of U(VI) by PGC microspheres conforms to the pseudo-second-order kinetic model and the Freundlich isothermal adsorption model. The adsorption mechanism of U(VI) by PGC microspheres is electrostatic interaction and chelation reaction. Meanwhile, PGC microspheres have excellent adsorption selectivity and recycling, and they have a good adsorption effect in the uranium treatment of rare earth real wastewater. Therefore, PGC microspheres can be used as a promising material to treat uranium-containing rare earth wastewater.
















Similar content being viewed by others
References
Michailidou G, Koumentakou I, Liakos EV et al (2021) Adsorption of uranium, mercury, and rare earth elements from aqueous solutions onto magnetic chitosan adsorbents: a review. Polymers (Basel) 13(18):3137
Jun BM, Parks Lee HK et al (2021) Purification of uranium-contaminated radioactive water by adsorption: a review on adsorbent materials. Sep Purif Technol 278:119675
Wang G, Liu J, Wang X et al (2009) Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J Hazard Mater 168(2–3):1053–1058
Kekeks T (2020) Adsorption of indigo carmine on functional chitosan and beta-cyclodextrin/chitosan beads: equilibrium, kinetics and mechanism studies. J Environ Manage 262:110372
GuoO H, Mei P (2021) Carbon materials for extraction of uranium from seawater. Chemosphere 278:130411
Pang H, Wu Y, Wang X et al (2019) Recent advances in composites of graphene and layered double hydroxides for Water remediation: a review. Chem Asian J 14(15):2542–2552
Liu X, Ma R, Wang X et al (2019) Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: a review. Environ Pollut 252(Pt A):62–73
Liu H, Zhou Y, Yang Y et al (2019) Synthesis of polyethylenimine/graphene oxide for the adsorption of U(VI) from aqueous solution. Appl Surf Sci 471:88–95
WANG F, LI H, LIU Q et al (2016) A graphene oxide/amidoxime hydrogel for enhanced uranium capture. Sci Rep 6:19367
Liu W, Wang Q, Wang H et al (2022) Adsorption of uranium by chitosan/Chlorella pyrenoidosa composite adsorbent bearing phosphate ligand. Chemosphere 287(Pt 2):132193
Senol ZM (2021) A chitosan-based composite for adsorption of uranyl ions; mechanism, isothems, kinetics and thermodynamics. Int J Biol Macromol 183:1640–1648
Bi C, Zhang C, Ma F et al (2022) Development of 3D porous ag + decorated PCN-222 @ graphene oxide-chitosan foam adsorbent with antibacterial property for recovering U(VI) from seawater. Sep Purif Technol 281:119900
Huang Z, Li Z (2017) Interaction mechanism of uranium(VI) with three-dimensional graphene oxide-chitosan composite: insights from batch experiments, IR, XPS, and EXAFS spectroscopy. Chem Eng J 328:1066–1074
Senol ZM, Kaya S (2022) Synthesis and characterization of chitosan-vermiculite-lignin ternary composite as an adsorbent for effective removal of uranyl ions from aqueous solution: experimental and theoretical analyses. Int J Biol Macromol 209(Pt A):1234–1247
Şenol ZM, Şimşek S (2022) Insights into effective adsorption of lead ions from Aqueous Solutions by using Chitosan-Bentonite Composite beads. J Polym Environ 30(9):3677–3687
AMPIAW RE (2020) Persimmon tannins as biosorbents for precious and heavy metal adsorption in wastewater: a review. Int J Environ Sci Technol 17(8):3835–3846
Santos SCR, Bacelo HA, Boaventura RA et al (2019) Tannin-adsorbents for Water Decontamination and for the recovery of critical Metals: current state and future perspectives. Biotechnol J 14(12):e1900060
Bacelo HAM, Santos SCR, Botelho CMS (2016) Tannin-based biosorbents for environmental applications: a review. Chem Eng J 303:575–587
LIU F, ZHOU Z (2021) Persimmon tannin functionalized polyacrylonitrile fiber for highly efficient and selective recovery of au(III) from aqueous solution [J]. Chemosphere 264(Pt 1):128469
Zhou S, Xie Y, Zhu F et al (2021) Amidoxime modified chitosan/graphene oxide composite for efficient adsorption of U(VI) from aqueous solutions. J Environ Chem Eng 9(6):106363
Guo Y, Liu X, Xie S (2022) 3D ZnO modified biochar-based hydrogels for removing U(VI) in aqueous solution. Colloids Surf A 642:128606
LI X, WANG Z, LIANG H et al (2019) Chitosan modification persimmon tannin bioadsorbent for highly efficient removal of pb(II) from aqueous environment: the adsorption equilibrium, kinetics and thermodynamics. Environ Technol 40(1):112–124
Dai Y, Zhou L, Tang X et al (2020) Macroporous ion-imprinted chitosan foams for the selective biosorption of U(VI) from aqueous solution. Int J Biol Macromol 164:4155–4164
Yang P, Liu Q, Liu J et al (2017) Interfacial growth of a metal–organic framework (UiO-66) on functionalized graphene oxide (GO) as a suitable seawater adsorbent for extraction of uranium(vi) [J]. J Mater Chem A 5(34):17933–17942
Wang Y, Lin Z, Liu Q et al (2021) Ultra-high mechanical property and multi-layer porous structure of amidoximation ethylene-acrylic acid copolymer balls for efficient and selective uranium adsorption from radioactive wastewater. Chemosphere 280:130722
NAN Y, WANG J, CHANG X et al (2023) Functionalized graphene oxide/sodium alginate beads with ion responsiveness for uranium trapping. Carbohydr Polym 300:120259
Zhuang S, Cheng R, Kang M et al (2018) Kinetic and equilibrium of U(VI) adsorption onto magnetic amidoxime-functionalized chitosan beads. J Clean Prod 188:655–661
KAYNAR Ü H, ÇıNAR S, ÇAM KAYNAR S et al (2017) Modelling and optimization of uranium (VI) ions adsorption onto Nano-ZnO/chitosan bio-composite beads with response surface methodology (RSM). J Polym Environ 26(6):2300–2310
HASAN S, GHOSH T K, PRELAS MA et al (2017) Adsorption of uranium on a novel bioadsorbent-chitosan-coated perlite. Nucl Technol 159(1):59–71
Basu H, Singhal RK, Pimple MV et al (2018) Graphene oxide encapsulated in alginate beads for enhanced sorption of uranium from different aquatic environments. J Environ Chem Eng 6(2):1625–1633
Liu L, Yang W (2019) In situ preparation of chitosan/ZIF-8 composite beads for highly efficient removal of U(VI). Front Chem 7:607
Cai Y, Wu C, Liu Z et al (2017) Fabrication of a phosphorylated graphene oxide–chitosan composite for highly effective and selective capture of U(VI). Environ Sci Nano 4(9):1876–1886
Xia M, Gao R, Xu G et al (2022) Fabrication and investigation of novel monochloroacetic acid fortified, tripolyphosphate-crosslinked chitosan for highly efficient adsorption of uranyl ions from radioactive effluents. J Hazard Mater 431:128461
WANG Z, LI X, LIANG H et al (2017) Equilibrium, kinetics and mechanism of au(3+), pd(2+) and ag(+) ions adsorption from aqueous solutions by graphene oxide functionalized persimmon tannin. Mater Sci Eng C Mater Biol Appl 79:227–236
Ahmad M, Ren J, Zhang Y et al (2022) Simple and facile preparation of tunable chitosan tubular nanocomposite microspheres for fast uranium(VI) removal from seawater. Chem Eng J 427:130934
GUO D, SONG X (2020) Recovery of uranium (VI) from aqueous solutions by the polyethyleneimine-functionalized reduced graphene oxide/molybdenum disulfide composition aerogels. J Taiwan Inst Chem Eng 106:198–205
GU H, JU P, LIU Q et al (2022) Constructing an amino-reinforced amidoxime swelling layer on a Polyacrylonitrile surface for enhanced uranium adsorption from seawater. J Colloid Interface Sci 610:1015–1026
Acknowledgements
This work was supported in part by the Natural Science Foundation of Hunan Province (No. 2021JJ30579); the Key Scientific Research Project of Education Bureau of Hunan Province (19A421).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, F., Huang, H., Sun, X. et al. Persimmon tannin-modified graphene oxide/chitosan microsphere for removing U(VI) in rare earth wastewater. J Radioanal Nucl Chem 332, 3617–3633 (2023). https://doi.org/10.1007/s10967-023-09032-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09032-x