Abstract
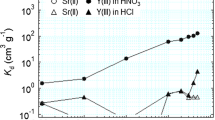
To achieve the mutual separation of yttrium(Y(III)) and strontium(Sr(II)) from acidic solution, a diglycolamic acid-grafted adsorbent (DGAA−Si adsorbent) was successfully developed via a facile amidation reaction between diglycolic anhydride and a 3-aminopropyl-functionalized silica-gel. The adsorption performances of DGAA−Si adsorbent toward Y(III) and Sr(II) were systematically investigated in terms of the effect of the acid concentration etc. The particle-induced X-ray emission analysis method was used to study the adsorption behavior in the solid-state and elucidated the distribution conditions of the adsorbed Y(III) and Sr(II). Moreover, the mutual separation of Y(III) and Sr(II) was successfully achieved using acid in different concentration as an effluent solution.





Similar content being viewed by others
References
Paik T, Chacko AM, Mikitsh JL, Friedberg JS, Pryma DA, Murray CB (2015) Shape-controlled synthesis of isotopic yttrium-90-labeled rare earth fluoride nanocrystals for multimodal imaging. ACS Nano 9:8718–8728
Vimalnath KV, Chakraborty S, Rajeswari A, Sarma HD, Nuwad J, Pandey U, Kamaleshwaran K, Shinto A, Dash A (2015) Radiochemistry, pre-clinical studies and first clinical investigation of 90Y-labeled hydroxyapatite (HA) particles prepared utilizing 90Y produced by (n, γ) route. Nucl Med Biol 42:455–464
Zhang ZJ, Igarashi JY, Satou Y, Ninomiya K, Sueki K, Shinohara A (2019) Activity of 90Sr in fallout particles collected in the difficult-to-return zone around the Fukushima Daiichi nuclear power plant. Environ Sci Technol 53:5868–5876
Chakravarty R, Chakraborty S, Jadhav S, Dash, (2019) A Facile radiochemical separation of clinical-grade 90Y from 90Sr by selective precipitation for targeted radionuclide therapy. Nucl Med Biol 68–69:58–65
Zou Y, Liang JC, Chu TW (2017) Crown ether–ionic liquid-based extraction chromatographic resin for separation of 90Y from 90Sr. J Radioanal Nucl Chem 311:1643–1648
Wood DJ, Elshani S, Du HS, Natale NR, Wai CM (1993) Separation of 90Y from 90Sr by solvent extraction with ionizable crown ethers. Anal Chem 65:1350–1354
Gujar RB, Ansari SA, Mohapatra PK (2015) Spectacular enhancements in actinide ion uptake using novel extraction chromatography resins containing TODGA and ionic liquid. Sep Purif Technol 141:229–234
Ogata T, Narita H, Tanaka M (2016) Adsorption mechanism of rare earth elements by adsorbents with diglycolamic acid ligands. Hydrometallurgy 163:156–160
Bai RX, Yang F, Zhang Y, Zhao ZG, Liao QX, Chen P, Zhao PP, Guo WH, Cai CQ (2018) Preparation of elastic diglycolamic-acid modified chitosan sponges and their application to recycling of rare-earth from waste phosphor powder. Carbohydr Polym 190:255–261
Wu H, Kim SY, Miwa M, Matsuyama S (2021) Synergistic adsorption behavior of a silica-based adsorbent toward palladium, molybdenum, and zirconium from simulated high-level liquid waste. J Hazard Mater 411:125136
Wu Y, Kim SY, Tozawa D, Ito T, Tada T, Hitomi K, Kuraoka E, Yamazaki H, Ishii K (2012) Study on selective separation of cesium from high level liquid waste using a macroporous silica-based supramolecular recognition absorbent. J Radioanal Nucl Chem 293:13–20
Matsuyama S, Hatakeyama T, Arai H, Kikuchi Y, Miwa M, Toyama S (2020) Development of 3D imaging systems using ion microbeams. Nuclear Inst Methods Phys Res B 482:31–36
Arshad F, Selvaraj M, Zain J, Banat F, Haij MA (2019) Polyethylenimine modified graphene oxide hydrogel composite as an efficient adsorbent for heavy metal ions. Sep Purif Technol 209:870–880
Kumar R, Sharma RK, Singh AP (2019) Grafting of cellulose with N-isopropylacrylamide and glycidyl methacrylate for efficient removal of Ni(II), Cu(II) and Pd(II) ions from aqueous solution. Sep Purif Technol 219:249–259
Choudhary BC, Paul D, Borsea AU, Garole DJ (2018) Surface functionalized biomass for adsorption and recovery of gold from electronic scrap and refinery wastewater. Sep Purif Technol 195:260–270
Ahmed IM, Hamed MM, Aglan RF, Aly MI (2019) Separation of strontium and yttrium in nitric acid solutions using zirconium titanium phosphate and Dowex exchangers. J Radioanal Nucl Chem 321:39–47
Vasylyeva H, Mironyuk I, Strilchuk M, Maliuk I, Savka K, Vasyliev O (2021) Adsorption and possibility of separation of heavy metal cations by strong cation exchange resin. Chem Phys 3:100056
Reza D, Elmira FA, Mohammad S, Maryam T, Parisa Z, Mojtaba S (2018) Selective separation of yttrium(III) through a liquid membrane system using 2-thenoyltrifuoroacetone as an extractant carrier. Chem Pap 72:1487–1497
Xu YL, Kim SY, Ito T, Nakazawa K, Funaki Y, Tada T, Hitomi K, Ishii K (2012) Adsorption and separation behavior of yttrium and strontium in nitric acid solution by extraction chromatography using a macroporous silica-based adsorbent. J Chromatogr A 1263:28–33
Kawamura T, Wu H, Kim SY (2021) Adsorption and separation behavior of strontium and yttrium using a silica-based bis(2-ethylhexyl) hydrogen phosphate adsorbent. J Radioanal Nucl Chem 329:1001–1009
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, SY., Kawamura, T. & Wu, H. Adsorption and separation of yttrium(III) and strontium(II) in acidic solutions using a diglycolamic acid-grafted adsorbent. J Radioanal Nucl Chem 332, 3001–3008 (2023). https://doi.org/10.1007/s10967-023-09010-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09010-3