Abstract
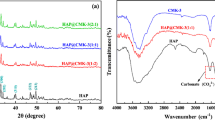
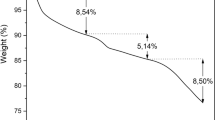
Hydroxyapatite Nano Rods (HAp-NR) was synthesized from Perna viridis shell bio-waste by chemical precipitation method followed by annealing with addition of poly vinyl alcohol (PVA). The formation of HAp-NR was confirmed by FTIR and XRD analysis. SEM and TEM images confirms rod like structure of HAp and the EDX studies have confirmed the presence of Ca, P and O with Ca/P ratio = 1.61 in HAp-NR. Removal of uranium (VI) from aqueous solution by batch adsorption experiments was investigated using HAp-NR. The influence of pH, contact time, initial U(VI) concentration, temperature and ionic strength on adsorption of U(VI) onto HAp-NR were carried out. The adsorption of U(VI) onto HAp-NR was confirmed by EDS elemental mapping, FTIR, and XRD studies. Kinetics of adsorption followed the Elovich model and the adsorption process reached equilibrium within 90 min. Adsorption isotherm data agreed well with the Langmuir isotherm model and HAp-NR exhibits maximum experimental adsorption capacity of 293.6 mg g−1 at 298 K. Temperature dependent adsorption studies reveals the endothermic nature of U(VI) adsorption onto HAp-NR. The adsorbent can be reused by leaching the adsorbed U(VI) by equilibrating with 0.05 M Na2CO3. Hence, the present study reveals that HAp-NR derived from Perna viridis shell bio-waste could be a potential simplistic nanomaterial for environmental remediation.














Similar content being viewed by others
References
Kim SK, Venkatesan J (2015) Introduction to marine biotechnology. In: Kim SK (ed) Springer handbook of marine biotechnology. Springer handbooks. Springer, Berlin. https://doi.org/10.1007/978-3-642-53971-8_1
Ibrahim A-R, Wei W, Zhang D, Wang H, Li J (2013) Conversion of waste eggshells to mesoporous hydroxyapatite nanoparticles with high surface area. Mater Lett 110:195–197. https://doi.org/10.1016/j.matlet.2013.08.014
Ducheyne P, Hench LL, Kagan A, Martens M, Bursens A, Mulier JC (1980) Effect of hydroxyapatite impregnation on skeletal bonding of porous coated implants. J Biomed Mater Res 14:225–237. https://doi.org/10.1002/jbm.820140305
Patel N, Brooks RA, Clarke MT, Lee PMT, Rushton N, Gibson IR, Best SM, Bonfield W (2005) In vivo assessment of hydroxyapatite and silicate-substituted hydroxyapatite granules using an ovine defect model. J Mater Sci - Mater Med 16:429–440. https://doi.org/10.1007/s10856-005-6983-6
Takikawa K, Akao M (1996) Fabrication of transparent hydroxyapatite and application to bone marrow derived cell/hydroxyapatite interaction observation in-vivo. J Mater Sci Mater Med 7:439–445. https://doi.org/10.1007/BF00122014
Ripamonti U, Crooks J, Khoali L, Roden L (2009) The induction of bone formation by coral-derived calcium carbonate/hydroxyapatite constructs. Biomaterials 30:1428–1439. https://doi.org/10.1016/j.biomaterials.2008.10.065
Ohare P, Meenan BJ, Burke GA, Byrne G, Dowling D, Hunt JA (2010) Biological responses to hydroxyapatite surfaces deposited via a co-incident microblasting technique”. Biomaterials 31:515–522. https://doi.org/10.1016/j.biomaterials.2009.09.067
Huber F-X, Berger I, McArthur N, Huber C, Kock H-P, Hillmeier J, Meeder P (2008) Evaluation of a novel nanocrystalline hydroxyapatite paste and a solid hydroxyapatite ceramic for the treatment of critical size bone defects (CSD) in rabbits. J Mater Sci Mater Med 19:33–38. https://doi.org/10.1007/s10856-007-3039-0
Neelakandeswari N, Sangami G, Dharmaraj N (2011) Preparation and characterization of nanostructured hydroxyapatite using a biomaterial. Synth React Inorg Met-Org Nano-Met Chem 41(5):513–516. https://doi.org/10.1080/15533174.2011.568434
Karunakaran G, Cho E-B, Kumar GS, Kolesnikov E, Janarthanan G, Pillai MM, Rajendran S, Boobalan S, Gorshenkov MV, Kuznetsov D (2019) Ascorbic acid-assisted microwave synthesis of mesoporous Ag-doped hydroxyapatite nanorods from biowaste seashells for implant applications. ACS Appl Bio Mater 2:2280–2293. https://doi.org/10.1021/acsabm.9b00239
Priyadarshini N, Sampath M, Kumar S, Mudali UK, Natarajan R (2013) A combined spectroscopic and light scattering study of hydrolysis of uranium(VI) leading to colloid formation in aqueous solutions. J Radioanal Nucl Chem 298:1923–1931. https://doi.org/10.1007/s10967-013-2624-6
Priyadarshini N, Benadict Rakesh K, Ilaiyaraja P (2018) Actinide separation in environment and their separation using functionalized nanomaterials and nanocomposits. In: Hussain CM (ed) Handbook of environmental materials management. Springer, Berlin, pp 19–20. https://doi.org/10.1007/978-3-319-58538-3-143-1
Rayno DR (1983) Estimated dose to man from Uranium milling via the beef/milk food-chain pathway. Sci Total Environ 31:219–241. https://doi.org/10.1016/0048-9697(83)90078-5
Kukkonen E, Virtanen EJ, Moilanen JO (2022) α-Aminophosphonates, phosphinates, and phosphine oxides as extraction and precipitation agents for rare earth metals, thorium, and uranium: a review. Molecules 27:3465. https://doi.org/10.3390/molecules27113465
Li S, Hu Y, Shen Z (2021) Rapid and selective uranium extraction from aqueous solution under visible light in the absence of solid photocatalyst. Sci China Chem 64:1323–1331. https://doi.org/10.1007/s11426-021-9987-1
Chen F, Lv M, Ye Y, Miao S, Tang X, Liu Y, Liang B, Qin Z, Chen Y, He Z, Wang Y (2022) Insights on uranium removal by ion exchange columns: the deactivation mechanisms, and an overlooked biological pathway. Chem Eng J 434:134708. https://doi.org/10.1016/j.cej.2022.134708
Chen T, Zhang J, Ge H, Li M, Li Y, Liu B, Duan T, He R, Zhu W (2020) Efficient extraction of uranium in organics-containing wastewater over g-C3N4/GO hybrid nanosheets with type-II band structure. J Hazard Mater 384:121383. https://doi.org/10.1016/j.jhazmat.2019.121383
Chen T, Yu K, Dong C, Yuan X, Gong X, Lian J, Cao X, Li M, Zhou L, Hu B, He R, Zhu W, Wang X (2022) Advanced photocatalysts for uranium extraction: elaborate design and future perspectives. Coord Chem Rev 467:214615. https://doi.org/10.1016/j.ccr.2022.214615
Yang L, Xiao H, Qian Y et al (2022) Bioinspired hierarchical porous membrane for efficient uranium extraction from seawater. Nat Sustain 5:71–80. https://doi.org/10.1038/s41893-021-00792-6
Yuan D, Zhao J, Zhang Q, Peng Lu, Wang Y, He Y, Liu Z, Liu Y, Zhao X, Meng C (2022) Highly efficient extraction of uranium from aqueous solution using imidazole functionalized core–shell sunflower-like superparamagnetic polymer microspheres: understanding adsorption and binding mechanisms. J Mater Chem A 10:12656–12668. https://doi.org/10.1039/D2TA02669D
Sahaa S, Basu H, Rout S, Pimple MV, Singhal RK (2020) Nano-hydroxyapatite coated activated carbon impregnated alginate: a new hybrid sorbent for uranium removal from potable water. J Environ Chem Eng 8:103999. https://doi.org/10.1016/j.jece.2020.103999
Su M, Tsang DCW, Ren X, Shi Q, Tang J, Zhang H, Kong L, Hou L, Song G, Chen D (2019) Removal of U(VI) from nuclear mining effluent by porous hydroxyapatite: evaluation on characteristics, mechanisms and performance. Environ Pollut 254:112891. https://doi.org/10.1016/j.envpol.2019.07.059
Yanhong Wu, Chen D, Kong L, Tsang DCW, Minhua Su (2019) Rapid and effective removal of uranium (VI) from aqueous solution by facile synthesized hierarchical hollow hydroxyapatite microspheres. J Hazard Mater 371:397–405. https://doi.org/10.1016/j.jhazmat.2019.02.110
Han T, Chen W, Cai Y, Lv Z, Zhang Y, Tan X (2022) Immobilization of uranium during the deposition of carbonated hydroxyapatite. J Taiwan Inst Chem Eng 134:104331. https://doi.org/10.1016/j.jtice.2022.104331
Zhou H, Xie Y, Wang X, Yang H, Wang Y, Zhang Y (2022) Efficient removal of uraniumin aqueous solution by Al-doped hydroxyapatite: static/dynamic adsorption behaviors and mechanism study. Environ Technol Innov 25:102103. https://doi.org/10.1016/j.eti.2021.102103
El-Maghrabi HH, Younes AA, Salem AR, Rabie K, El-Shereafy E-S (2019) Magnetically modified hydroxyapatite nanoparticles for the removal of uranium (VI): Preparation, characterization and adsorption optimization. J Hazard Mater 378:120703. https://doi.org/10.1016/j.jhazmat.2019.05.096
Xiong T, Jia L, Li Q, Zhang Y, Zhu W (2022) Efficient removal of uranium by hydroxyapatite modified kaolin aerogel. Sep Purif Technol 299:121776. https://doi.org/10.1016/j.seppur.2022.121776
Tang M, Shen J, Xia X, Jin B, Chen K, Zeng T (2022) A novel microbial induced synthesis of hydroxyapatite with highly efficient adsorption of uranyl(VI). Colloids Surf A Physicochem Eng Asp 635:128046. https://doi.org/10.1016/j.colsurfa.2021.128046
Xuan K, Wang J, Gong Z, Wang X, Li J, Guo Y, Sun Z (2022) Hydroxyapatite modified ZIF-67 composite with abundant binding groups for the highly efficient and selective elimination of uranium (VI) from wastewater. J Hazard Mater 426:127834. https://doi.org/10.1016/j.jhazmat.2021.127834
Guo Y, Gong Z, Li C, Gao B, Li P, Wang X, Zhang B, Li X (2020) Efficient removal of uranium (VI) by 3D hierarchical Mg/Fe-LDH supported nanoscale hydroxyapatite: a synthetic experimental and mechanism studies. Chem Eng J 392:123682. https://doi.org/10.1016/j.cej.2019.123682
Suresh Kumar C, Dhanaraj K, Vimalathithan RM, Ilaiyaraja P, Suresh G (2021) Structural, morphological and anti-bacterial analysis of nanohydroxyapatite derived from biogenic (SHELL) and chemical source: formation of apatite. Iran J Mater Sci Eng 18:91–109. https://doi.org/10.22068/ijmse.18.1.10
Dhanaraj K, Suresh G (2018) Conversion of waste sea shell (Anadara granosa) into valuable nanohydroxyapatite (nHAp) for biomedical applications. Vaccum 152:222–230. https://doi.org/10.1016/j.vacuum.2018.03.021
Savvin S (1961) Analytical use of arsenazo III Determination of thorium zirconium, uranium and rare earth elements. Talanta 8:673–685. https://doi.org/10.1016/0039-9140(61)80164-1
Sadar SM, Khorasani MT, Dinpanah-Khoshdargi E, Jamshidi A (2013) Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater 9:7591–7662. https://doi.org/10.1016/j.actbio.2013.04.012
Dauphin Y, Denis A (2000) Structure and composition of the aragonite crossed lamellar layers in six species of Bivalvia and Gastropoda. Comp Biochem Physiol Part A 126:367–377. https://doi.org/10.1016/s1095-6433(00)00213-0
Nunez D, Elgueta E, Varaprasad K, Oyarzun P (2018) Hydroxyapatite nanocrystals synthesized from calcium rich bio-wastes. Mater Lett 230:64–68. https://doi.org/10.1016/j.matlet.2018.07.077
Suresh Kumar C, Dhanaraj K, Vimalathithan RM, Ilaiyaraja P, Suresh G (2020) Hydroxyapatite for bone related applications derived from sea shell waste by simple precipitation method. J Asian Ceram Soc 8(2):416–429. https://doi.org/10.1080/21870764.2020.1749373
Dhanaraj K, Suresh Kumar C, Socrates SH, Vinoth Arulraj J, Suresh G (2021) A comparative analysis of microwave assisted natural (Murex virgineus shell) and chemical nanohydroxyapatite: structural, morphological and biological studies. J Aust Ceram Soc 57:173–183. https://doi.org/10.1007/s41779-020-00522-9
Suresh Kumar G, Girija EK, Venkatesh M, Karunakaran G, Evgeny Kolesnikov E, Kuznetsov D (2017) One step method to synthesize flower-like hydroxyapatite architecture using mussel shell bio-waste as a calcium source. Ceram Int 43(3):3457–3461. https://doi.org/10.1016/j.ceramint.2016.11.163
Bensalah H, Bekheet MF, Younssi SA, Ouammou M, Gurlo A (2018) Hydrothermal synthesis of nanocrystalline hydroxyapatite from phosphogypsum waste. J Environ Chem Eng 6:1347–1352. https://doi.org/10.1016/j.jece.2018.01.052
Shanmugam N, Dhanaraj K, Viruthagiri G, Balamurugan K, Deivam K (2016) Synthesis and characterization of surfactant, assisted Mn2+ doped ZnO nanocrystals. Arab J Chem 9:S758–S764. https://doi.org/10.1016/j.arabjc.2011.08.016
Guan Y, Cao W, Wang X, Marchetti A, Tu Y (2018) Hydroxyapatite nano-rods for the fast removal of Congo red dye from aqueous solution. Mater Res Express 5:065053. https://doi.org/10.1088/2053-1591/aaccb8
Sadat-Shojai M, Khorasani MT, Dinpanah-Khoshdargi E, Jamshidi A (2013) Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater 9:7591–7621. https://doi.org/10.1016/j.actbio.2013.04.012
Mehta D, Mondal P, Saharan VK, George S (2017) Synthesis of Hydroxyapatite Nanorods for application in water defluoridation and optimization of process variables: advantage of ultrasonication with precipitation method over conventional method. Ultrason Sonochem 37:56–70. https://doi.org/10.1016/j.ultsonch.2016.12.035
Dotto GL, Pinto LAA (2011) Adsorption of food dyes onto chitosan: optimization process and kinetic. Carbohyd Polym 84:231–238. https://doi.org/10.1016/j.carbpol.2010.11.028
He Y, Wang Y, Cai C et al (2023) Cotton stalk derived carbon pretreated by microbial fermentation for selective uranium extraction. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-023-08827-2
Sakr AK, Abdel Aal MM, Abd El-Rahem KA, Allam EM, Abdel Dayem SM, Elshehy EA, Hanfi MY, Alqahtani MS, Cheira MF (2022) Characteristic aspects of uranium(VI) adsorption utilizing nano-silica/chitosan from wastewater solution. Nanomaterials 12:3866. https://doi.org/10.3390/nano12213866
Liao J, Xiong T, Ding L, Xie Y, Zhang Y, Zhu W (2022) Design of a renewable hydroxyapatite-biocarbon composite for the removal of uranium(VI) with high-efficiency adsorption performance. Biochar 4:29. https://doi.org/10.1007/s42773-022-00154-1
Ilaiyaraja P, Singha Deb AK, Ponraju D, Musharaf Ali SK, Venkatraman B (2017) Surface engineering of PAMAM-SDB chelating resin with diglycolamic acid (DGA) functional group for efficient sorption of U(VI) and Th(IV) from aqueous medium. J Hazard Mater 328:1–11. https://doi.org/10.1016/j.jhazmat.2017.01.001
Acknowledgements
The authors sincerely thank school of advanced sciences, Vellore Institute of Technology Chennai for providing necessary infrastructure and characterization techniques to carry out this work.
Funding
All authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that no conflicts of interest for this research manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhanasekaran, A., Suresh, G., Perumal, I. et al. Bio-waste derived hydroxyapatite nano rods for U(VI) uptake from aqueous medium. J Radioanal Nucl Chem 332, 3235–3247 (2023). https://doi.org/10.1007/s10967-023-08976-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08976-4