Abstract
Sustainable development of nuclear energy benefits from high-quality host materials that treat high-level waste simply and safely. A natural aluminosilicate was used to immobilize CeO2 in a simulating diagenetic process. Below 7 wt% of CeO2, cerium exists in glass in the forms of Si–O–Ce and gap-filling. As it exceeds 7 wt%, being promoted by Ce4+ reduction, uncured cerium nucleates on the bubble wall. Sample with 7 wt% of CeO2 shows higher hardness, density and lower normalized leaching rate (10−5 g m2 d−1 after 28 d), indicating an excellent stability. This study proposes the natural aluminosilicate as a promising material for treating high-level waste.
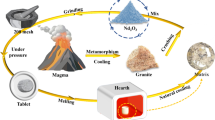
Graphical abstract















Similar content being viewed by others
References
Ramana MV (2018) Technical and social problems of nuclear waste. Wiley Interdiscip Rev Energy Environ 7(4):289
Kerr RA (2000) Nuclear waste disposal: science and policy clash at Yucca Mountain. Science 288(5466):602–602
Silverio LB, Lamas WQ (2011) An analysis of development and research on spent nuclear fuel reprocessing. Energ Policy 39(1):281–289
Chung Y, Park D, Kim H et al (2020) The impact of gamma-irradiation from radioactive liquid wastewater on polymeric structures of nanofiltration (NF) membranes. J Hazard Mater 403:123578–123578
Sengupta P, Fanara S, Fanara S (2011) Preliminary study on calcium aluminosilicate glass as a potential host matrix for radioactive 90Sr–An approach based on natural analogue study. J Hazard Mater 190(1–3):229–239
Losq CL, Valentine AP, Mysen BO et al (2021) Structure and properties of alkali aluminosilicate glasses and melts: insights from deep learning. Geochim Cosmochim Ac 314:27–54
Hwa LG, Lee TH, Szu SP (2004) Elastic properties of lanthanum aluminosilicate glasses. Mater Res Bull 39(1):33–40
Cormier L, Delbes L, Baptiste B et al (2021) Vitrification, crystallization behavior and structure of zinc aluminosilicate glasses. J Non Cryst Solids 555:120609
Zhao J, Nienhuis ET, McCloy JS et al (2020) (2020) Structures of fluoride containing aluminosilicate low activity nuclear waste glasses: a molecular dynamics simulations study. J Non Cryst Solids 550:120379
Li L, Shu X, Cheng Y et al (2022) High immobilizing capacity of natural granite as glass-ceramic matrix to simulated trivalent actinide waste. Radiat Phys Chem 195:110067
Guo-Malloy S, McMillan PF, Petuskey WT (2016) Glass formation and characterization in the 3Al2O3·2SiO2–LaPO4 system. J Non Cryst Solids 451:77–83
Atila A, Ghardi EM, Ouaskit S et al (2019) Atomistic insights into the impact of charge balancing cations on the structure and properties of aluminosilicate glasses. Phys Rev B 100(14):144109
Shi C, Fernández-Jiménez A (2006) Stabilization/solidification of hazardous and radioactive wastes with alkali-activated cements. J Hazard Mater 137(3):1656–1663
Mysen B (2021) Structure of aluminosilicate melts. ISIJ Int 61(12):2866–2881
Hassaanl MY, Saudi HA, El-Bahnasawy HH et al (2021) Preparation and characterization of egyptian granite based glass with different Na+ ions content. SILICON. https://doi.org/10.1007/s12633-021-01249-3
Eilaghi M, Montazerian M, Yekta BE (2016) Effect of partial substitution of K2O for Na2O on sintering, crystallization and mechanical properties of SiO2–CaO–K2O–Na2O–CaF2 glass-ceramics. T Indian Ceram Soc 75(1):1–6
Pickering JM, Johnston DA (1998) Fluid-absent melting behavior of a two-mica metapelite: experimental constraints on the origin of black hills granite. J Petrol 39(10):1787–8104
Nardi LVS, Bonina B (1991) Post-orogenic and non-orogenic alkaline granite associations: the Saibro intrusive suite, southern Brazil-a case study. Chem Geol 92(1–3):197–211
Lu X, Chen S, Shu X et al (2018) Immobilisation of nuclear waste by microwave sintering with a natural magmatic rock. Philos Mag Lett 98(4):155–160
Li Y, Yu H, Zheng L et al (2013) Compressive strength of fly ash magnesium oxychloride cement containing granite wastes. Constr Build Mater 38:1–7
Freire-Lista DM, Fort R, Varas-Muriel MJ (2016) Thermal stress-induced microcracking in building granite. Eng Geol 206:83–93
Zhu R, Lai S, Qin J et al (2020) Genesis of high-potassium calc-alkaline peraluminous I-type granite: new insights from the Gaoligong belt granites in southeastern Tibet Plateau. Lithos 354:105343
Zhu H, Wang F, Liao Q et al (2020) Effect of CeO2 and Nd2O3 on phases, microstructure and aqueous chemical durability of borosilicate glass-ceramics for nuclear waste immobilization. Mater Chem Phys 249:122936
Tan P, Shu X, Wen M (2002) Characteristics of cerium doped aluminosilicate glass as simulated radioactive waste forms: effect on structures and properties. Prog Nucl Energ 150:104299
Kajdas B, Michalik MJ, Migoń P (2017) Mechanisms of granite alteration into grus, Karkonosze granite, SW Poland. CATENA 150:230–245
Pozo-Antonio JS, Sanmartín P, Serrano M et al (2020) Impact of wildfire on granite outcrops in archaeological sites surrounded by different types of vegetation. Sci Total Environ 747:141143
Li H, Wang R (1997) The age the Fenghuangshan granite resolved by U-Pb single zircon. Prog Precambrian Res 20(3):56–62
Krynine PD (1948) The megascopic study and field classification of sedimentary rocks. J Geol 56(2):130–165
Guo M, Yin X, Du X (2023) Effect of aging, testing temperature and relative humidity on adhesion between asphalt binder and mineral aggregate. Constr Build Mater 363:129775
Luo Y, Wang F, Zhu H (2022) Preparation and characterization of glass-ceramics with granite tailings and titanium-bearing blast furnace slags. J Non-Cryst Solids 582:121463
Verma SP, Torres-Alvarado IS, Sotelo-Rodrı́guez ZT (2000) SINCLAS: standard igneous norm and volcanic rock classification system. Comput Geosci-UK 28(5):711–715
Wang M, Zhang S, Yang Z et al (2022) Sintering behaviors and thermal properties of Li2SiO3-based ceramics for LTCC applications. Ceram Int 48(19):27312–27323
Strachan DM, Turcotte RP, Barnes BO (1982) MCC-1: a standard leach test for nuclear waste forms. Nucl Technol 56(2):306–312
American Society for Testing and Materials (ASTM) C1220 (2020) Standard test method for static leaching of monolithic waste forms for disposal of radioactive waste
Zhu H, Wang F, Liao Q et al (2019) (2019) Structure features, crystallization kinetics, and water resistance of borosilicate glasses doped with CeO2. J Non Cryst Solids 518:57–65
Zhu W, Chen X, Struble L et al (2018) Characterization of calcium-containing phases in alkali-activated municipal solid waste incineration bottom ash binder through chemical extraction and deconvoluted Fourier transform infrared spectra. J Clean Prod 18:782–789
Hu Q, Suzuki H, Gao H et al (2003) High-frequency FTIR absorption of SiO2/Si nanowires. Chem Phys Lett 378(3–4):299–304
Zhang Y, Shan L, Tu Z et al (2008) Preparation and characterization of novel Ce-doped nonstoichiometric nanosilica/polysulfone composite membranes. Sep Purif Technol 63:207–212
Tao Y, Tao YX, Su ZH et al (2013) Shape-controlled CeO2 nanomaterials prepared by hydrothermal. Appl Mech Mater 331:513–521
Liu H, Li S, Wu F et al (2016) Effect of different Ca/La ratio on structure and properties of Al–B–Si glass with low dielectric constant. J Sci-Mater El 27(9):9821–9827
Zhang L, Qu Y, Wan X et al (2020) Influence of rare earth oxides on structure, dielectric properties and viscosity of alkali-free aluminoborosilicate glasses. J Non Cryst Solids 532:119886
Kang J, Chen J, Li S et al (2020) Structure, dielectric property and viscosity of alkali-free boroaluminosilicate glasses with the substitution of Al2O3 for SiO2. J Non Cryst Solids 537:120022
Sun Y, Zhang Z (2015) Structural roles of boron and silicon in the CaO–SiO2–B2O3 glasses using FTIR, Raman, and NMR spectroscopy. Metall Mater Trans B 46(4):1549–1554
Geng X, Cao J, Wang Z et al (2020) Selective controlled precipitation mechanism of canasite and xonotlite in glass-ceramics from silica slag. J Non Cryst Solids 546:120283
El-Damrawi G, Abd-El-Nour K, Ramadan RM (2018) Structural and dielectric studies on Na2O–PbO–SiO2 glasses. SILICON 11:495–500
Thomas B, Prathapan S, Sugunan S (2005) Solid acid-catalyzed dehydration/beckmann rearrangement of aldoximes: towards high atom efficiency green processes. Micropor Mesopor Mat 79(1–3):21–27
Kaya H, Ngo D, Gin S et al (2022) Spectral changes in Si–O–Si stretching band of porous glass network upon ingress of water. J Non Cryst Solids 527:119722
Wacławska I, Szumera M, Sułowska J (2016) Structural characterization of zinc-modified glasses from the SiO2–P2O5–K2O–CaO–MgO system. J Alloy Compd 666:352–358
Karakassides MA, Saranti A, Koutselas I (2004) Preparation and structural study of binary phosphate glasses with high calcium and/or magnesium content. J Non Cryst Solids 347:69–79
Slebarski A, Deniszczyk J, Kaczorowski D (2020) Mixed valence of Ce and its consequences on the magnetic state of Ce9Ru4Ga5: electronic structure studies. Materials 13(10):2377
Beche E, Peraudeau G, Flaud V et al (2012) An XPS investigation of (La2O3)1–x (CeO2)2x (ZrO2)2 compounds. Surf Interface Anal 44(8):1045–1050
Feng Y, Chen Q, Zhou Y et al (2020) Modification of glass structure via CaO addition in granulated copper slag to enhance its pozzolanic activity. Constr Build Mater 240:117970
Huang Q, Liu J, He X et al (2021) Analysis of structure evolution and performance in alkali-free glass substrates via XPS and infrared: Boron-aluminum anomaly. J Non Cryst Solids 555:120531
Jaiswal SK, Hong J, Yoon KJ et al (2016) Optical absorption and XPS studies of (Ba1–xSrx) (Ce0.75 Zr0.10y0.15) o 3-δ electrolytes for protonic ceramic fuel cells. Ceram Int 42(8):10366–10372
John S (2017) Glass-ceramics for nuclear-waste immobilization. MRS Bull 42(3):233–240
Zhu H, Wang F, Liao Q et al (2020) Synthesis and characterization of zirconolite-sodium borosilicate glass-ceramics for nuclear waste immobilization. J Nucl Mater 532:152026
Du Y, Han S, Zou Y et al (2019) Luminescence properties of Ce3+ doped oxyfluoride aluminosilicate glass and glass ceramics. Opt Mater 89:243–249
Wang HM, Henderson GS (2008) O K-edge XANES studies of alkali germanophosphate glasses. J Non Cryst Solids 354(10–11):864–872
Wang Z, Zhao Z, Peng B et al (2019) Investigation on the mechanism of the immobilization of CeO2 by using cullet-based glass (CBG). Ann Nucl Energy 133:209–215
Yang D, Zhao B, Chen B et al (2017) Study on impurities of ZnGeP2 single crystal and its effect on infrared optical property. Mater Res Express 4(7):075906
Li S, Lu Y, Qu Y et al (2021) Dielectric and thermal properties of aluminoborosilicate glasses doped with mixed rare-earth oxides. J Non-Cryst Solids 556:120550
Zhao J, Lu Y, Kang J et al (2018) Effect of Y2O3 and La2O3 on structure and dielectric properties of aluminoborosilicate glasses. J Non-Cryst Solids 496:1–5
Xiong L, Earl RA (2009) MoO3 and Sb2O3 effects on bubble formation in silicate glass coatings during sintering. Mater Lett 63:360–362
Weinberg MC, Onorato PIK, Uhlmann DR (1980) Behavior of bubbles in glassmelts: dissolution of a stationary bubble containing a single gas. J Am Ceram Soc 63(3–4):175–180
Shevchenko VY, Glushkova VB, Panova TI et al (2001) Preparation of ultrafine tetragonal ZrO2–CeO2 solid solutions. Inorg mater 37(7):692–697
Davis MJ, Ihinger PD (1998) Heterogeneous crystal nucleation on bubbles in silicate melt. Am Miner 83(9–10):1008–1015
Gualda GAR, Ghiorso MS, Ghiorso MS (2007) Magnetite scavenging and the buoyancy of bubbles in magmas. Part 2: energetics of crystal-bubble attachment in magmas. Contrib Miner Petr 154(4):479–490
Zhang H, Suzuki-Muresan T, Gin S et al (2023) Effects of vapor hydration and radiation on the leaching behavior of nuclear glass. J Nucl Mater 578:154368
Zhang Q, Wang C, Zhang H et al (2020) Designing ultrahard nanostructured diamond through internal defects and interface engineering at different length scales. Carbon 170:94–402
Abou Hussein EM, Abdel Maksoud MIA, Fahim RA et al (2021) Unveiling the gamma irradiation effects on linear and nonlinear optical properties of CeO2–Na2O–SrO–B2O3 glass. Opt Mater 114:111007
Duh JG, Dai HT (1988) Sintering, microstructure, hardness, and fracture toughness behavior of Y2O–CeO–ZrO. J Am Ceram Soc 71(10):813–819
Choi HS, Im HN, Kim YM et al (2016) Structural, thermal, and mechanical properties of aluminum nitride ceramics with CeO2 as a sintering aid. Ceram Int 42(10):11519–11524
Shu X, Huang W, Shi K et al (2021) Microwave vitrification of simulated radioactively contaminated soil: mechanism and performance. J Solid State Chem 293:121757
Luo S, Xu Z, Liu J et al (2021) The solubility, microstructure, and chemical durability of Ce-doped YIG ceramics designed as actinide waste forms. Int J Energ Res 45(14):19883–19884
Wen G, Zhang K, Zhang H et al (2015) Immobilization and aqueous durability of Nd2O3 and CeO2 incorporation into rutile TiO2. Ceram Int 41:6869–6875
Acknowledgements
The authors appreciate the supports from the National Natural Science Foundation of China (No. 21976146, No. U2267219), Sichuang Natural Science Foundation Project “Study on the composition and properties of high entropy ceramic solidified body based on TRPO waste” (No. 23NSFSC0991), National Health Commission Nuclear Technology Medical Transformation Key Laboratory Annual Open Project (No. 2021HYX028), the Project of State Key Laboratory of Environment-friendly Energy Materials, Southwest University of Science and Technology (No. 22fksy12), the Applied Basic Research Project of Science and Technology Department of Sichuan Province (No. 2021YJ0344) and National College Students Innovation and Entrepreneurship Training Program (No. 202210619016).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, L., Shu, X., Tan, P. et al. Role of radioactive waste on microstructure evolution and performance in natural aluminosilicate system. J Radioanal Nucl Chem 332, 2653–2666 (2023). https://doi.org/10.1007/s10967-023-08938-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08938-w



