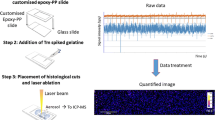
Abstract
The spatial distribution of thorium, uranium and other radioactive elements in biological tissues is significant for evaluating their migration, toxicity and possible decorporation mechanism, however, it is still a big challenge today for developing quantitative imaging by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) due to the lack of matrix matched standards for calibration. Herein, a fast and reliable LA-ICP-MS quantitative imaging method was developed by using gelatin plus standard solution to prepare a self-made reference material containing 100 μg/g of U and Th, overcoming the inhomogeneity of the raw animal tissue standard. Then the elemental spatial distribution of kidney and liver tissues from mice were revealed by using LA-ICP-MS method. The results showed that U accumulated mainly in the kidney and slightly in the liver, while most of Th was distributed in the liver and few of that in kidney. It was found that U and Th were heterogeneously distributed in kidney section while homogeneously distributed in liver section.








Similar content being viewed by others

References
Pereira R, Barbosa S, Carvalho FP (2014) Uranium mining in Portugal: a review of the environmental legacies of the largest mines and environmental and human health impacts. Environ Geochem Health 36:285–301. https://doi.org/10.1007/s10653-013-9563-6
Hao Y, Ren J, Liu J, Yang Z, Liu C, Li R, Su Y (2013) Immunological changes of chronic oral exposure to depleted uranium in mice. Toxicology 309:81–90. https://doi.org/10.1016/j.tox.2013.04.013
Chaudhury D, Sen U, Sahoo BK, Bhat NN, Kumara KS, Karunakara N, Biswas S, Shenoy PS, Bose B (2022) Thorium promotes lung, liver and kidney damage in BALB/c mouse via alterations in antioxidant systems. Chem Biol Interact 363:109977. https://doi.org/10.1016/j.cbi.2022.109977
Francischini DS, Arruda MAZ (2021) When a picture is worth a thousand words: molecular and elemental imaging applied to environmental analysis—A review. Microchem J 169:106526. https://doi.org/10.1016/j.microc.2021.106526
Doble PA, de Vega RG, Bishop DP, Hare DJ, Clases D (2021) Laser ablation-inductively coupled Pplasma-mass spectrometry imaging in biology. Chem Rev 121:11769–11822. https://doi.org/10.1021/acs.chemrev.0c01219
Homma-Takeda S, Uehara A, Yoshida T, Numako C, Sekizawa O, Nitta K, Sato N (2020) Two-dimensional μXAFS analysis for accumulated uranium in kidneys of rats exposed to uranyl acetate. Radiat Phys Chem 175:108147. https://doi.org/10.1016/j.radphyschem.2019.02.006
Tessier C, Suhard D, Rebiere F, Souidi M, Dublineau I, Agarande M (2012) Uranium microdistribution in renal cortex of rats after chronic exposure: a study by secondary ion mass spectrometry microscopy. Microsc Microanal 18:123–133. https://doi.org/10.1017/S1431927611012384
Homma-Takeda S, Fujishiro H, Tanaka I, Yakumaru H, Ayama K, Uehara A, Oikawa M, Himeno S, Ishihara H (2021) Single-cell imaging for studies of renal uranium transport and intracellular behavior. Minerals 11:191. https://doi.org/10.3390/min11020191
Grijalba N, Legrand A, Holler V, Bouvier-Capely C (2020) A novel calibration strategy based on internal standard-spiked gelatine for quantitative bio-imaging by LA-ICP-MS: application to renal localization and quantification of uranium. Anal Bioanal Chem 412:3113–3122. https://doi.org/10.1007/s00216-020-02561-4
Togao M, Nakayama SMM, Ikenaka Y, Mizukawa H, Makino Y, Kubota A, Matsukawa T, Yokoyama K, Hirata T, Ishizuka M (2020) Bioimaging of Pb and STIM1 in mice liver, kidney and brain using Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) and immunohistochemistry. Chemosphere 238:124581. https://doi.org/10.1016/j.chemosphere.2019.124581
Halbach K, Wagner S, Scholz S, Luckenbach T, Reemtsma T (2019) Elemental imaging (LA-ICP-MS) of zebrafish embryos to study the toxicokinetics of the acetylcholinesterase inhibitor naled. Anal Bioanal Chem 411:617–627. https://doi.org/10.1007/s00216-018-1471-2
Jurowski K, Buszewski B, Piekoszewski W (2015) The analytical calibration in (bio)imaging/mapping of the metallic elements in biological samples–definitions, nomenclature and strategies: state of the art. Talanta 131:273–285. https://doi.org/10.1016/j.talanta.2014.07.089
Pessoa GS, Lopes Junior CA, Madrid KC, Arruda MAZ (2017) A quantitative approach for Cd, Cu, Fe and Mn through laser ablation imaging for evaluating the translocation and accumulation of metals in sunflower seeds. Talanta 167:317–324. https://doi.org/10.1016/j.talanta.2017.02.029
Ralbovsky NM, Zou L, Chen B, Zhang NR, Hines CDG, Vavrek M, Zhong W, Smith JP, Bu X (2021) Simultaneous multielement imaging of liver tissue using laser ablation inductively coupled plasma mass spectrometry. Talanta 235:122725. https://doi.org/10.1016/j.talanta.2021.122725
VanderSchee CR, Kuter D, Chou H, Jackson BP, Mann KK, Bohle DS (2020) Addressing K/L-edge overlap in elemental analysis from micro-X-ray fluorescence: bioimaging of tungsten and zinc in bone tissue using synchrotron radiation and laser ablation inductively coupled plasma mass spectrometry. Anal Bioanal Chem 412:259–265. https://doi.org/10.1007/s00216-019-02244-9
Weller A, Zok D, Steinhauser G (2019) Uptake and elemental distribution of radiosilver 108mAg and radiocesium 137Cs in shiitake mushrooms (Lentinula edodes). J Radioanal Nucl Chem 322:1761–1769. https://doi.org/10.1007/s10967-019-06778-1
Neves VM, Heidrich GM, Rodrigues ES, Enders MSP, Muller EI, Nicoloso FT, Carvalho HWPd, Dressler VL (2019) La2O3 nanoparticles: study of uptake and distribution in Pfaffia glomerata (Spreng.) Pedersen by LA-ICP-MS and μ-XRF. Environ Sci Technol 53:10827–10834. https://doi.org/10.1021/acs.est.9b02868
Jim V, LaViolette C, Briehl MM, Ingram JC (2017) Spatial distribution of uranium in mice kidneys detected by laser ablation inductively coupled plasma mass spectrometry. J Appl Bioanal 3:43–48. https://doi.org/10.17145/jab.17.007
Sala M, Selih VS, van Elteren JT (2017) Gelatin gels as multi-element calibration standards in LA-ICP-MS bioimaging: fabrication of homogeneous standards and microhomogeneity testing. Analyst 142:3356–3359. https://doi.org/10.1039/c7an01361b
Zhou J, Ni X, Fu J, Li Y, Guo W, Jin L, Hu S (2021) Quantitation and imaging analysis of biological samples by LA-ICP-MS. Atomic Spectroscopy 42(4):210–216. https://doi.org/10.46770/as.2021.068
Villaseñor Á, Greatti C, Boccongelli M, Todolí JL (2017) A dried droplet calibration approach for the analysis of solid samples through laser ablation—inductively coupled plasma mass spectrometry. J Anal Atom Spectrom 32:587–596. https://doi.org/10.1039/c6ja00343e
Moreno-Gordaliza E, Giesen C, Lazaro A, Esteban-Fernandez D, Humanes B, Canas B, Panne U, Tejedor A, Jakubowski N, Gomez-Gomez MM (2011) Elemental bioimaging in kidney by LA-ICP-MS as a tool to study nephrotoxicity and renal protective strategies in cisplatin therapies. Anal Chem 83:7933–7940. https://doi.org/10.1021/ac201933x
Lear J, Hare D, Adlard P, Finkelstein D, Doble P (2012) Improving acquisition times of elemental bio-imaging for quadrupole-based LA-ICP-MS. J Anal Spectrom 27:159–164. https://doi.org/10.1039/c1ja10301f
Cole LM (2017) Imaging mass spectrometry. Name Humana Press, New York
Vaculovič T, Warchilová T, Čadková Z, Száková J, Tlustoš P, Otruba V, Kanický V (2015) Influence of laser ablation parameters on trueness of imaging. Appl Surf Sci 351:296–302. https://doi.org/10.1016/j.apsusc.2015.05.136
Li Q, Fang Y, Liu J, Zhang C, Wang Z (2021) Elemental imaging of alumina ceramic tube using laser ablation- inductively coupled plasma-mass spectrometry (LA-ICP-MS). Atom Spectrosc 42:154–159. https://doi.org/10.46770/as.2021.019
Berradi H, Bertho J-M, Dudoignon N, Mazur A, Grandcolas L, Baudelin C, Grison S, Voisin P, Gourmelon P, Dublineau I (2008) Renal anemia induced by chronic ingestion of depleted uranium in rats. Toxicol Sci 103:397–408. https://doi.org/10.1093/toxsci/kfn052
Donnadieu-Claraz M, Bonnehorgne M, Dhieux B, Maubert C, Cheynet M, Paquet F, Gourmelon P (2007) Chronic exposure to uranium leads to iron accumulation in rat kidney cells. Radiat Res 167:454–464. https://doi.org/10.1667/RR0545.1
Terada Y, Homma-Takeda S, Takeuchi A, Suzuki Y (2010) High-energy X-Ray microprobe system with submicron resolution for X-Ray fluorescence analysis of uranium in biological specimens. X-Ray Opt Instrument 2010:1–5. https://doi.org/10.1155/2010/317909
Li C, Xu W, Chu S, Zheng Z, Xiao Y, Li L, Bi H, Wei L (2018) The chemical speciation, spatial distribution and toxicity of mercury from Tibetan medicine Zuotaibeta-HgS and HgCl2 in mouse kidney. J Trace Elem Med Biol 45:104–113. https://doi.org/10.1016/j.jtemb.2017.08.010
Frick DA, Günther D (2012) Fundamental studies on the ablation behaviour of carbon in LA-ICP-MS with respect to the suitability as internal standard. J Anal Atom Spectrom 27:1294. https://doi.org/10.1039/c2ja30072a
Sabolić I (2006) Common mechanisms in nephropathy induced by toxic metals. Nephron Physiol 104:107–114. https://doi.org/10.1159/000095539
Homma-Takeda S, Terada Y, Nakata A, Sahoo SK, Yoshida S, Ueno S, Inoue M, Iso H, Ishikawa T, Konishi T, Imaseki H, Shimada Y (2009) Elemental imaging of kidneys of adult rats exposed to uranium acetate. Nucl Instrum Meth B 267:2167–2170. https://doi.org/10.1016/j.nimb.2009.03.082
Homma-Takeda S, Kokubo T, Terada Y, Suzuki K, Ueno S, Hayao T, Inoue T, Kitahara K, Blyth BJ, Nishimura M, Shimada Y (2013) Uranium dynamics and developmental sensitivity in rat kidney. J Appl Toxicol 33:685–694. https://doi.org/10.1002/jat.2870
Homma-Takeda S, Numako C, Kitahara K, Yoshida T, Oikawa M, Terada Y, Kokubo T, Shimada Y (2019) Phosphorus localization and its involvement in the formation of concentrated uranium in the renal proximal tubules of rats exposed to uranyl acetate. Int J Mol Sci 20:4677. https://doi.org/10.3390/ijms20194677
Acknowledgements
This work was supported by the Transformational Technologies for Clean Energy and Demonstration Strategic Priority Research Program of the Chinese Academy of Sciences (XDA21000000), DNL Cooperation Fund, CAS (Grant No. DNL202008), the National Science Foundation of China (Grant No. 22179141), and Youth Innovation Promotion Association, Chinese Academy of Science (Grant No. 2022257).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Wang, X., Zhou, J. et al. Bioimaging of uranium and thorium in mice organs by laser ablation inductively coupled plasma mass spectrometry. J Radioanal Nucl Chem 332, 2559–2569 (2023). https://doi.org/10.1007/s10967-023-08932-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08932-2