Abstract
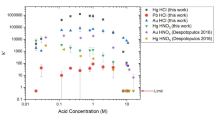
The uptake behavior of 73As and 75Se was studied in HCl and HNO3-H2O2 solutions on commercial extraction chromatography resins (TEVA, TRU, DGA, and Pb resin). There was no uptake of 73As or 75Se from HNO3 media. From HCl, there was 75Se uptake at high concentrations on all the resins and no uptake of 73As. Separations of 75Se and 73As on these resins have high yields and high radiopurity for 73As, but limited recovery of 75Se. TRU and TEVA resin may have potential for use in isotope generators as 75Se can be retained for at least 26 days with repeated elutions.






Similar content being viewed by others
References
Jennewein M, Lewis MA, Zhao D, Tsyganov E, Slavine N, He J, Watkins L, Kodibagkar VD, O’Kelly S, Kulkarni P, Antich PP, Hermanne A (2008) Vascular imaging of solid tumors in rats with a radioactive arsenic-labeled antibody that binds exposed phosphatidylserine. Clin Cancer Res 14(5):1377–1385
Mandal A, Lahiri S (2012) Production and separation of no-carrier-added 73As and 75Se from 7Li irradiated germanium oxide target. Radiochim Acta 100:856–870
Wycoff DE, Gott MD, DeGraffenreid AJ, Morrow RP, Sisay N, Embree MF, Ballard B, Fassbender ME, Cutler CS, Ketring AR, Jurisson SS (2014) Chromatographic separation of selenium and arsenic: a potential 72Se/72As generator. J Chromatogr A 1340:109–114
Chattopadhyay S, Pal S, Vimalnath KV, Das MK (2007) A versatile technique for radiochemical separation of medically useful no-carrier-added (nca) radioarsenic from irradiated germanium oxide targets. Appl Radiat Isot 65(11):1202–1207
Kelley K, Hoffman R, Dietrich F, Mustafa M (2006) Neutron Induced Cross sections for Radiochemistry for Isotopes of Arsenic. Lawrence Livermore National Laboratory UCRL-TR-218181
Hanson S, Oldham W (2021) Weapons Radiochemistry: Trinity and Beyond. Nucl Technol 207:S295–S308
Siri S, Segovia MS, Cohen IM (2019) The production of no carrier added arsenic radioisotopes in nuclear reactors. J Radioanal Nucl Chem 319:175–184
Jahn M, Radchenko V, Filosofov DV, Hauser H, Eisenhut M, Rosch F, Jennewein M (2010) Separation and purification of no-carrier-added arsenic from bulk amounts of germanium for use in radiopharmaceutical labelling. Radiochim Acta 98:807–812
Mukhopadhyay K, Nayak D, Lahiri S (2002) Separation of no-carrier-added as and Se produced in 16O irradiated cobalt target. J Radioanal Nucl Chem 251(1):159–162
Nayak D, Lahiri S (2003) Sequential separation of 61Cu, 62,63Zn, 66,67,68Ga, 71,72As, and 73Se produced by heavy ion activation on Cobalt Target. J Nucl Radiochem Sci 4(1):1–3
Feng Y, Phipps MD, Phelps TE, Okoya NC, Baumeister JE, Wycoff DE, Dorman EF, Wooten AL, Vlasenko V, Berendzen AF, Wilbur DS, Hoffman TJ, Cutler CS, Ketring AR, Jurisson SS (2019) Evaluation of 72Se/72As generator and production of 72Se for supplying 72As as a potential PET imaging radionuclide. Appl Radiat Isot 143:113–122
Chajduk E, Doner K, Polkowska-Motrenko H, Bilewicz A (2012) Novel radiochemical separation of arsenic from selenium for 72Se/72As generator. Appl Radiat Isot 70:819–822
Ballard B, Wycoff D, Birnbaum ER, John KD, Lenz JW, Jurisson SS, Cutler CS, Nortier FM, Taylor WA, Fassbender ME (2012) Selenium-72 formation via natBr(p,x) induced by 100 MeV protons: steps towards a novel 72Se/72As generator system. Appl Radiat Isot 70:595–601
Zhu Z, Sasaki Y, Suzuki H, Suzuki S, Kimura T (2004) Cumulative study on solvent extraction of elements by N,N,N′,N′-tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid into n-dodecane. Anal Chim Acta 527(2):163–168
Aprahamian VH, Demopoulos GP (1995) The Solution Chemistry and Solvent extraction Behaviour of Cu, Fe, Ni, Zn, Pb, Sn, Ag, as, Sb, Bi, Se and Te in Acid Chloride Solutions reviewed from the standpoint of PGM Refining. Miner Process Extr Metall Rev 14(3–4):143–167
Chowdhury MR, Sanyal SK (1993) Separation by solvent extraction of tellurium(IV) and selenium(IV) with tri-n butyl phosphate: some mechanistic aspects. Hydrometallurgy 32(2):189–200
Nyaba L, Matong JM, Dimpe KM, Nomngongo PN (2016) Speciation of inorganic selenium in environmental samples after suspended dispersive solid phase microextraction combined with inductively coupled plasma spectrometric determination. Talanta 159:174–180
Mafu LD, Msagati TAM, Mamba BB (2014) The simultaneous stripping of arsenic and selenium from wastewaters using hollow-fibre supported liquid membranes. Environ Monit Assess 186:8865–8874
Leddicotte G (1961) The Radiochemistry of Selenium. Subcommittee on Radiochemistry-National Academy of Sciences, Oak Ridge, TN
“DGA Resins” (2022) Eichrom Technologies. https://www.eichrom.com/eichrom/products/dga-resins/. Accessed 1 Nov 2022
Kimura K (1960) Inorganic extraction studies on the system between bis (2-ethyl hexyl)-orthophosphoric acid and hydrochloric acid (I). Bull Chem Soc Jpn 33(8):1038–1046
Horwitz EP, Chiarizia R, Dietz ML, Diamond H, Nelson DM (1993) Separation and preconcentration of actinides from acidic media by extraction chromatography. Anal Chim Acta 281(2):361–372
Horwitz EP, Dietz ML, Chiarizia R, Diamond H, Maxwell SL, Nelson M (1995) Separation and preconcentration of actinides by extraction chromatography using a supported liquid anion exchanger: application to the characterization of high-level nuclear waste solutions. Anal Chim Acta 310:63–78
Horwitz EP, McAlister DR, Bond AH, Barrans RE (2005) Novel extraction Chromatographic Resins based on tetraalkyldiglycolamides: characterization and potential applications. Solvent Extr Ion Exch 23(3):319–344
Horwitz EP, Gale NH (1994) A lead-selective extraction Chromatographic Resin and its application to the isolation of lead from geological samples. Anal Chim Acta 292:263–273
Horwitz EP, Dietz ML, Nelson DM, LaRosa JJ, Fairman WD (1990) Concentration and separation of actinides from urine using a supported bifunctional organophosphorus extractant. Anal Chim Acta 238:263–271
National Nuclear Data Center (2019) Brookhaven National Laboratory. https://www.nndc.bnl.gov/nudat2/indx_dec.jsp. Accessed 6 Dec 2022
Horwitz EP, Dietz M, Chiarizia R, Diamond H, Essling A, Graczyk D (1992) Separation and preconcentration of uranium from acidic media by extraction chromatography. Anal Chim Acta 266:25–37
Horwitz EP, Chiarizia R, Dietz M (1992) A novel strontium-selective extraction chromatographic resin. Solvent Extr Ion Exch 10:313–336
Stewart II, Chow A (1993) The separation of tellurium and selenium by polyurethane foam sorbents. Talanta 40(9):1345–1352
Marin L, Lhomme J, Carignan J (2001) Determination of Selenium Concentration in Sixty five reference materials for geochemical analysis by GFAAS after separation with Thiol Cotton. Geostand Geoanal Res 25(2–3):317–324
Kmak KN, Despotopulos JD, Scielzo ND (2023) Extraction of selenium and arsenic with TOA-impregnated XAD-2 resin from HCl. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-023-08818-3
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Cryst A 32:751–767
Despotopulos JD, Kmak KN, Gharibyan N, Henderson RA, Moody KJ, Shaughnessy DA, Sudowe R (2016) Characterization of the homologs of flerovium with crown ether based extraction chromatography resins: studies in hydrochloric acid. J Radioanal Nucl Chem 310:1201–1207
Thakur P, Ward AL (2020) 210Po in the environment: insight into the naturally occurring polonium isotope. J Radioanal Nucl Chem 323:27–49
Acknowledgements
This study was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344. This work was funded by the Laboratory Directed Research and Development Program at LLNL under project tracking code 23-SI-004.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kmak, K.N., Despotopulos, J.D. & Scielzo, N.D. Behavior of selenium and arsenic in HCl and HNO3 on TRU, TEVA, DGA, and Pb extraction chromatography resins. J Radioanal Nucl Chem 332, 1647–1655 (2023). https://doi.org/10.1007/s10967-023-08904-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08904-6