Abstract
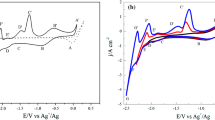
In order to improve the utilization of spent fuel, electrolysis is used to recover fissile elements from nuclear waste samarium. The electrochemical behaviors of Sm(III) and Pb(II) were examined by CV, SWV, and OCP techniques in LiCl–KCl molten salts at 773 K. The redox mechanism of Sm(III) ions on the liquid Pb thin film electrode was analyzed. The results demonstrate that Sm(III) electro-reduction at the inert W electrode only occurred in a soluble–soluble electrochemical transition Sm(III)/Sm(II). Additionally, the kinetic parameters of samarium and lead ions were computed. The activation energies of diffusion for Sm(III) and Pb(II) were 32.92 kJ mol−1 and 35.21 kJ mol−1, respectively. SmPb3 and Sm2Pb intermetallic compounds were derived using constant potential electrolysis and constant current electrolysis at the Pb electrode in a molten salt system. The cathodic deposition output was characterized by XRD and SEM–EDS. The ICP-AES results showed that the average extraction ratio of Sm on the Pb electrode was about 94.23%.


















Similar content being viewed by others
References
Han W, Wang W, Li M, Wang J, Sun Y, Yang X, Zhang M (2020) Electrochemical behavior and extraction of zirconium on Sn-coated W electrode in LiCl–KCl melts. Sep Purif Technol 232:1159651. https://doi.org/10.1016/j.seppur.2019.115965
Bruno JD, Ewing RC (2006) Spent nuclear fuel. Elements 2(6):343–349. https://doi.org/10.2113/gselements.2.6.343
Wang YC, Liu Q, Zhang S, Liu YH, Wang YQ, Dai Y, Dong ZM, Cheng ZP, Cao XH, Chen YQ, Zhang ZB, Liu YH (2022) Electrolytic extraction of yttrium using recycle liquid gallium electrode from molten LiCl–KCl. Sep Purif Technol 294:120972. https://doi.org/10.1016/j.seppur.2022.120972
Zhang J (2014) Electrochemistry of actinides and fission products in molten salts-data review. J Nucl Mater 447:271–284. https://doi.org/10.1016/j.jnucmat.2013.12.017
Taylor R (2015) Reprocessing and recycling of spent nuclear fuel. Elsevier, Amsterdam
Liu YL, Yuan LY, Kui L, Ye GA, Zhang ML, He H, Tang HB, Lin RS, Chai ZF, Shi WQ (2014) Electrochemical extraction of samarium from LiCl–KCl melt by forming Sm–Zn alloys. Electrochim Acta 120:369–378. https://doi.org/10.1016/j.electacta.2013.12.081
Wang YC, Liu Q, Quan MY, Liu YH, Chen YQ, Ren P, Zhang ZB, Liu YH (2022) Efficient recovery of the fission element neodymium by electrodeposition from molten LiCl–KCl and research on the thermodynamics and dynamics of processes. ACS Sustain Chem Eng 10:12796–12807. https://doi.org/10.1021/acssuschemeng.2c03712
Moriyama HYH, Nishikawa S et al (1998) Thermodynamics of reductive extraction of actinides and lanthanides from molten chloride salt into liquid metal. J Alloys Compd 271:587–591
Han W, Wang F, Tian Y, Zhang M, Yan Y (2011) Electrochemical formation of Mg–Li–Sm alloys by codeposition from LiCl–KCl–MgCl2–SmCl3 molten salts. Metall Mater Trans B 42:1376–1382. https://doi.org/10.1007/s11663-011-9550-1
Li S, Che Y, Li C, Shu Y, He J, Yang B, Song J (2022) Study on the electrochemical behavior of Mg and Al ions in LiCl–KCl melt and preparation of Mg–Al alloy. J Magn Alloys 10:721–729. https://doi.org/10.1016/j.jma.2020.06.020
Wang Y, Li M, Han W, Zhang M, Yang Y, Sun Y, Zhao Y, Yan Y (2015) Electrochemical extraction and separation of praseodymium and erbium on reactive magnesium electrode in molten salts. J Solid State Electrochem 19:3629–3638. https://doi.org/10.1007/s10008-015-2989-2
Cassayre L, Caravaca C, Jardin R, Malmbeck R, Masset P, Mendes E, Serp J, Soucek P, Glatz JP (2008) On the formation of U–Al alloys in the molten LiCl–KCl eutectic. J Nucl Mater 378:79–85. https://doi.org/10.1016/j.jnucmat.2008.05.004
Wang L, Liu YL, Liu K, Tang SL, Yuan LY, Su LL, Chai ZF, Shi WQ (2014) Electrochemical extraction of cerium from CeO2 assisted by AlCl3 in molten LiCl–KCl. Electrochim Acta 147:385–391. https://doi.org/10.1016/j.electacta.2014.08.113
Yan YD, Li X, Zhang ML, Xue Y, Tang H, Han W, Zhang ZJ (2012) Electrochemical extraction of ytterbium and formation of Al–Yb alloy from Yb2O3 assisted by AlCl3 in LiCl–KCl melt. J Electrochem Soc 159:D649–D655. https://doi.org/10.1149/2.049211jes
Yin TQ, Chen L, Xue Y, Zheng YH, Wang XP, Yan YD, Zhang ML, Wang GL, Gao F, Qiu M (2020) Electrochemical behavior and underpotential deposition of Sm on reactive electrodes (Al, Ni, Cu and Zn) in a LiCl–KCl melt. Int J Min Metals Mater 27:1657–1665. https://doi.org/10.1007/s12613-020-2112-2
Liao CF, Jiao YF, Wang X, Cai BQ, Sun QC, Tang H (2017) Electrical conductivity optimization of the Na3AlF6–Al2O3–Sm2O3 molten salts system for Al–Sm intermediate binary alloy production. Int J Min Metals Mater 24:1034–1042. https://doi.org/10.1007/s12613-017-1493-3
Karfidov EA, Nikitina EV (2019) Corrosion electrochemical behavior of nickel in the LiCl–KCl melt containing lanthanum trichloride. Russ Metall 2019:820–824. https://doi.org/10.1134/s003602951908007x
Liu Y, Zhang S, Zhong W, Cui G, Wang Y, Dai Y, Cao X, Wang Y, Zhang Z, Liu Y (2019) Electrochemical extraction of Sm(III) on active Ni electrode fabricated Sm–Ni alloys. J Radioanal Nucl Chem 322:1003–1010. https://doi.org/10.1007/s10967-019-06775-4
Ding L, Yan Y, Smolenski V, Novoselova A, Xue Y, Ma F, Zhang M (2021) Electrochemical studies based on the extraction of Zr on Cu electrode in the LiCl–KCl molten salt. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2021.119683
Ge J, Yang Q, Wang Y, Zhuo W, Du M, Zhang J (2020) Selective electrodeposition of europium and samarium in molten LiCl–KCl with copper and aluminum electrodes. J Electrochem Soc. https://doi.org/10.1149/1945-7111/ab628a
Li M, Liu B, Ji N, Sun Y, Han W, Jiang T, Peng S, Yan Y, Zhang M (2016) Electrochemical extracting variable valence ytterbium from LiCl–KCl–YbCl3 melt on Cu e-lectrode. Electrochim Acta 193:54–62. https://doi.org/10.1016/j.electacta.2016.02.020
Wang Y, Liu Y, Li M, Han W, Liu Y, Liu Y (2018) Extraction of gadolinium on Cu electrode from LiCl–KCl melts by formation of Cu–Gd alloys. Ionics 25:1897–1909. https://doi.org/10.1007/s11581-018-2762-5
Ji N, Huang W, Han W (2021) Electrochemical behavior of Tb(III) in LiCl–KCl molten salt On liquid Zn electrode. J Electrochem Soc 168:092503. https://doi.org/10.1149/1945-7111/ac2689
Li W, Han W, Li M, Zhang Y, Zhang Y, Yue M, Sun Y (2020) Electroreduction of Dy(III) assisted by Zn and its co-deposition with Zn(II) in LiCl–KCl molten salt. Appl Organomet Chem. https://doi.org/10.1002/aoc.5817
Wang L, Liu YL, Liu K, Tang SL, Yuan LY, Lu T, Chai ZF, Shi WQ (2015) Electrochemical extraction of cerium by forming Ce–Zn alloys in LiCl–KCl eutectic on W and liquid Zn electrodes. J Electrochem Soc 162:E179–E184. https://doi.org/10.1149/2.1141509jes
Li B, Liu K, Pang J, Yuan L, Liu Y, Lin M (2018) Electrochemical properties of gadolinium on liquid gallium electrode in LiCl–KCl eutectic. J Rare Earth 36:656–661. https://doi.org/10.1016/j.jre.2017.11.014
Li S, Liu Y, Che Y, Song J, Shu Y, He J, Xu B, Yang B (2020) Recycling of spent Indium–Gallium–Zinc oxide based on molten salt electrolysis. ACS Sustain Chem Eng 8:16296–16303. https://doi.org/10.1021/acssuschemeng.0c05986
Novoselova A, Smolenski V (2020) Solubility and activity coefficients of uranium in bimetallic Ga–In and Ga–Al liquid alloys. J Radioanal Nucl Chem 326:621–626. https://doi.org/10.1007/s10967-020-07313-3
Soldatova MN, Maltsev DS, Volkovich VA (2020) Separation of uranium and zirconium in a “chloride melt Ga–Zn eutectic alloy” system. AIP Conference Proceedings 2312. https://doi.org/10.1063/5.0032190
Han W, Wang W, Zhang Y, Wang Y, Li M, Sun Y (2021) Electrode reaction of Pr on Sn electrode and its electrochemical recovery from LiCl–KCl molten salt. Int J Energ Res 45:8577–8592. https://doi.org/10.1002/er.6394
Li M, Sun Z, Han W, Sun Y, Yang X, Wang W (2020) Electrochemical reaction of Sm(III) on liquid Sn electrode. J Electrochem Soc. https://doi.org/10.1149/1945-7111/ab6285
Han W, Li W, Chen J, Li M, Li Z, Dong Y, Zhang M (2019) Electrochemical properties of yttrium on W and Pb electrodes in LiCl–KCl eutectic melts. RSC Adv 9:26718–26728. https://doi.org/10.1039/c9ra05496k
Han W, Wang W, Li M, Wang D, Li H, Chen J, Sun Y (2021) Electrochemical separation of La from LiCl–KCl fused salt by forming La–Pb alloys. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2021.119188
Li Z, Liu Z, Li W, Han W, Li M, Zhang M (2020) Electrochemical recovery of dysprosium from LiCl–KCl melt aided by liquid Pb metal. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2020.117124
Li Z, Tang D, Meng S, Gu L, Dai Y, Liu Z (2021) Electrolytic separation of Dy from Sm in molten LiCl–KCl using Pb–Bi eutectic alloy cathode. Sep Purif Technol 276:119045. https://doi.org/10.1016/j.seppur.2021.119045
Quan MY, Liu Q, Liu YH, Zhang ZB, Dai Y, Wang YQ, Cao XH, Cheng ZP, Wang YC, Liu YH (2022) Electroextraction and thermochemistry of fission element gadolinium on plumbum electrode in molten salt. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2021.119413
Han W, Li Z, Li M, Li W, Zhang M, Yang X, Sun Y (2019) Reductive extraction of lanthanides (Ce, Sm) and its monitoring in LiCl–KCl/Bi–Li system. J Nucl Mater 514:311–320. https://doi.org/10.1016/j.jnucmat.2018.12.010
Kim B K PBG, Han HJ, et al (2018) An effect of bismuth ion on the reduction of terbium ion in molten LiCl–KCl eutectic salt. In: International conference on nuclear engineering American society of mechanical engineers 51517:V007T010A022.
Yin T, Liu Y, Jiang S, Yan Y, Wang G, Chai Z, Shi W (2021) Kinetic properties and electrochemical separation of uranium on liquid bismuth electrode in LiCl–KCl melt. J Electrochem Soc. https://doi.org/10.1149/1945-7111/abeb28
Lv A (1979) Evaluation of the selectivity of electrochemical reactor-fuel recovery on the basis of thermodynamic data. Soviet Atomic Energy 47(43):731–733
Yin TQ, Xue Y, Yan YD, Ma ZC, Ma FQ, Zhang ML, Wang GL, Qiu M (2021) Recovery and separation of rare earth elements by molten salt electrolysis. Int J Min Metals Mater 28:899–914. https://doi.org/10.1007/s12613-020-2228-4
Castrillejo Y, Fernández P, Medina J, Hernández P, Barrado E (2011) Electrochemical extraction of samarium from molten chlorides in pyrochemical processes. Electrochim Acta 56:8638–8644. https://doi.org/10.1016/j.electacta.2011.07.059
Osteryoung JGORA (1985) Square wave voltammetry. Anal Chem 57(51):101–110
Ramaley LKMS (1969) Theory of square wave voltammetry. Anal Chem 41(11):1362–1365
Shaltry MR, Allahar KN, Butt DP, Simpson MF, Phongikaroon S (2020) Electrochemical impedance spectroscopy and cyclic voltammetry methods for monitoring SmCl3 concentration in molten eutectic LiCl–KCl. J Nuclear Fuel Cycle Waste Technol 18:1–18. https://doi.org/10.7733/jnfcwt.2020.18.1.1
Han W, Wang WJ, Dong YC, Li M, Yang XG, Zhang ML (2018) The kinetics process of a Pb(ii)/Pb(0) couple and selective fabrication of Li–Pb alloys in LiCl–KCl melts. RSC Adv 8(53):30530–30538. https://doi.org/10.1039/C8RA06329J
Vandarkuzhali S, Gogoi N, Ghosh S, Prabhakara Reddy B, Nagarajan K (2012) Electrochemical behaviour of LaCl3 at tungsten and aluminium cathodes in LiCl–KCl eutectic melt. Electrochim Acta 59:245–255. https://doi.org/10.1016/j.electacta.2011.10.062
Zhao J, Liu Z, Liang W, Lu G (2022) Evaluation of the local structure and electrochemical behavior in the LiCl–KCl–SmCl3 melt. J Mol Liq. https://doi.org/10.1016/j.molliq.2022.119818
Acknowledgements
The work was financially supported by the National Natural Science Foundation of China, Supported by the Opening Project of Jiangxi Province Key Laboratory of Polymer Micro/Nano Manufacturing and Devices (Nos. U2167223, 22076022 and PMND202206).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Shen, Y., Zhang, Y. et al. Electrochemical separation of fission element samarium on lead electrodes from KCl–LiCl molten salts and the kinetic research of the process. J Radioanal Nucl Chem 332, 1353–1365 (2023). https://doi.org/10.1007/s10967-023-08815-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08815-6