Abstract
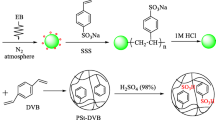
This work describes the investigation of applicability of Sulfonated Glycidylmethacrylate (GMA) grafted non-woven polyethylene (PE) adsorbent for the efficient removal of uranium from aqueous solutions. The adsorbent film was prepared by the gamma radiation (pre-radiation) technique of GMA grafting onto non-woven PE and the epoxide group of GMA converted to sulfonic acid by sulfonation. The uranium loading capacity of the sorbent was investigated with different parameters contact time, initial uranium concentration, pH and temperature. The kinetics of the monolayer sorption was evaluated by pseudo-first-order and pseudo-second-order equations. The uranium loading capacity depends on temperature. At 25 °C the value of ∆G is − 0.74 kJ mol−1 and at higher temperature it is also negative, indicates that the sorption process is spontaneous and thermodynamically favorable. The experimental data fitted best with the Langmuir isotherm model and the deliberate sorption capacity were 181.82 mg g−1.












Similar content being viewed by others
Data availability
All data of this work is given in manuscript figures and tables.
References
Penalonga LR, Soria BYM (2017) A review of the nuclear fuel cycle strategies and the spent nuclear fuel management technologies. Energies 10(8):1235
Wrenn ME, Durbin PW, Willis DL et al (1987) The potential toxicity of uranium in water. Am Water Works Assoc J 79:177–184
BEIR IV (1988) Health risk of radon and other internally deposited alpha-emitters. In: Biological effects of ionizing radiations, National Academy Press. Washington, pp 276–302
Xiaoliang S, Gongwang Q (2004) The hazard of the radioactive contamination and the preventive methods. Ind Saf Dust Control 30(1):6–9
Li ZJ et al (2017) Enhanced photocatalytic removal of uranium(VI) from aqueous solution by magnetic TiO2/Fe3O4 and its graphene composite. Environ Sci Technol 51:5666–5674
Wang L et al (2017) Rational control of the interlayer space inside two-dimensional titanium carbides for highly efficient uranium removal and imprisonment. Chem Commun 53:12084–12087
Tan LJ et al (2015) Facile preparation of oxine functionalized magnetic Fe3O4 particles for enhanced uranium (VI) adsorption. Coll Surf A Physicochem Eng Asp 466:85–91
Lingamdinne LP et al (2017) Preparation and characterization of porous reduced graphene oxide based inverse spinel nickel ferrite nanocomposite for adsorption removal of radionuclides. J Hazard Mater 326:145–156
Decker JD et al (2017) Ship-in-a-bottle CMPO in MIL-101(Cr) for selective uranium recovery from aqueous streams through adsorption. J Hazard Mater 335:1–9
Shen J, Schafer A (2014) Removal of fluoride and uranium by nanofiltration and reverse osmosis: a review. Chemosphere 117:679–691
Sreenivas T, Rajan KC (2013) Studies on the separation of dissolved uranium from alkaline carbonate leach slurries by resin-in-pulp process. Separ Purif Technol 112:54–60
Metwally SS, Ahmed IM, Rizk HE (2017) Modification of hydroxyapatite for removal of cesium and strontium ions from aqueous solution. J Alloy Compd. 709:438–444
Zhang C, Dodge CJ, Malhotra SV, Francis AJ (2013) Bioreduction and precipitation of uranium in ionic liquid aqueous solution by Clostridium sp. Bioresour Technol 136:752–756
Dworjanyn PA, Garnett JL (1988) Synergistic effects of urea with polyfunctional acrylates for enhancing the photografting of styrene to polypropylene. J Polym Chem Polym Lett Ed 26:135
Hollahan JR (1974) Plasma chemistry industrial application. Wiley, New York, p 113
Wilkie CA, Deacon C (1996) Graft copolymerization of acrylic acid on to acrylonitrile-butadiene-styrene terpolymer and thermal analysis of the copolymersur. Polym J 32:451
Nasef M, Saidi H, Dahlan KM, Chinese J (2010) Radiation grafted poly (vinylidene fluoride)-graft-polystyrene sulfonic acid membranes for fuel cells: structure-property relationships. Polym Sci 28(5):761
Hsieh L, Shinawatra YM, Castillo MD (1986) Postirradiation polymerization of vinyl monomers on poly(ethylene terephthalate). J Appl Polym Sci 31:509–519
Bari EMA, Sarhan AA, Razik HHA (1986) Effect of graft copolymerization of 2-hydroxyethyl methacrylate on the properties of polyester fibers and fabric. J Appl Polym Sci 35:439–448
Nasef MM (2000) Gamma radiation-induced graft copolymerization of styrene onto poly (ethylene terephthalate) films. J Appl Polym Sci 77:1003–1012
Bai J, Ma X, Yan H, Zhu J, Wang K, Wang J (2020) A novel functional porous organic polymer for the removal of uranium from wastewater. Microp Mesop Mater 306:110441
Yang Z, Chen G, Weng H, Shen W, Huang Z, Lin M (2020) Efficient and selecting separation of U(VI) and Th(IV) from rare earths using functionalized hierarchically mesoporous silica. J Mater Sci 53:3398–3416. https://doi.org/10.1007/s10853-017-1808-9,CASArticleGoogleScholar
Jiang D, Liu L, Pan N, Yang F, Li S, Wang R, Wyman IW, Jin Y, Xia C (2015) The separation of Th(IV)/U(VI) via selective complexation with graphene oxide. Chem Eng J 271:147–154. https://doi.org/10.1016/j.cej.2015.02.066,CASArticleGoogleScholar
Salah BA, Gaber MS, Kandil AT (2019) The removal of uranium and thorium from their aqueous solutions by 8-hydroxyquinoline immobilized bentonite. Minerals 9:626. https://doi.org/10.3390/min9100626
Ang KL, Li D, Nikoloski AN (2017) The effectiveness of ion exchange resins in separating uranium and thorium from rare earth elements in acidic aqueous sulfate media. Part 1 Anionic and cationic Resins. Hydrometallurgy 174:147–155. https://doi.org/10.1016/j.hydromet.2017.10.011
Dacrory S, Haggag EA, Masoud AM, Abdo SM, Eliwa AA, Kamel S (2020) Innovative synthesis of modified cellulose derivative as a uranium adsorbent from carbonate solutions of radioactive deposits. Cellulose 27:7093–7108. https://doi.org/10.1007/s10570-020-03272-w,CASArticleGoogleScholar
Alqadami AA, Naushad Mu, Alothman ZA, Ghfar AA (2017) Novel metal–organic framework (MOF) based composite material for the sequestration of U(VI) and Th(IV) metal ions from aqueous environment. ACS Appl Mater Interfaces 9:36026–36037. https://doi.org/10.1021/acsami.7b10768
Zhou L, Jia Y, Peng J, Liu Z, Al-Zaini E (2014) Competitive adsorption of uranium(VI) and thorium(IV) ions from aqueous solution using triphosphate-crosslinked magnetic chitosan resins. J Radioanal Nucl Chem 302:331–340. https://doi.org/10.1007/s10967-014-3125-y
Ke X, Drache M, Gohs U, Kunz U, Beuermann S (2018) Preparation of polymer electrolyte membranes via radiation-induced graft copolymerization on poly(ethylene-alttetrafluoroethylene) (ETFE) using the crosslinker N, N′-methylenebis (acrylamide). Membranes 8(4):102. https://doi.org/10.3390/membranes8040102
Md SR, Nazia R, Tofail AC, Nirmal CD, Shahnaz S, Md NS, Md NK (2021) Application of sulfonated GMA-g-non woven PE fabric for the efficient removal of methylene blue dye from wastewater. Am J Polym Sci Technol 7(1):1–9. https://doi.org/10.11648/j.ajpst.20210701.11
Md S R, Nazia R, Tofail AC, Nirmal CD, Shahnaz S, Md NS, Md NK Synthesis of sulfonated glycidylmethacrylate grafted non woven polyethylene adsorbent and investigation of its applicability for the efficient and recyclable adsorption of heavy metals from aqueous solutions. Taylor and Francis, submitt J. Plast rubber compos, Article no 222634040
Acknowledgements
We are happy to acknowledge IAEA to carry out the research for technical support and very grateful to Gamma source, Division of Institute of Food and Radiation and Radiation Biology, Atomic Energy Research Establishment.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, experimental work, data collection and analysis were performed by MSR, NR and SS. Figure was prepared by MSR and MNS. The initial draft of the manuscript were written by MSR and MNK and all authors commented on previous versions of the Manuscript.” Manuscript was finally edited by TAC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rana, M.S., Rahman, N., Chowdhury, T.A. et al. Investigations of applicability of sulfonated-GMA-g-non-woven PE adsorbent for the efficient removal of uranium from aqueous solutions. J Radioanal Nucl Chem 332, 737–746 (2023). https://doi.org/10.1007/s10967-023-08802-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08802-x