Abstract
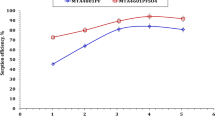
Efficient removal of iodide (I−) and iodate (IO3−) from aqueous solution is highly desirable for waste disposal and remediation of radioiodine contamination. We report here a bifunctional anion exchange resin Purolite A530E for the effective I−/IO3− adsorption, which shows a priority anion-exchange affinity for I− (99%) over IO3− (9.7%). The maximum adsorption capacity for I− is 275.16 mg·g−1 fitted by the Langmuir isotherm model, much higher than most adsorbents. Furthermore, Purolite A530E exhibits great selectivity for I− over competing anions, which endows the resin with promising I− remediation that over 99% I− can be removed from simulated Hanford groundwater (HGW).







Similar content being viewed by others
References
Riley BJ, Vienna JD, Strachan DM, McCloy JS, Jerden JL (2016) Materials and processes for the effective capture and immobilization of radioiodine: A review. J Nucl Mater 470:307–326
Huve J, Ryzhikov A, Nouali H, Lalia V, Auge G, Daou TJ (2018) Porous sorbents for the capture of radioactive iodine compounds: a review. RSC Adv 8:29248–29273
Hirota M, Higaki S, Ito S, Ishida Y, Terao K (2021) Effects of 2-hydroxypropyl α-cyclodextrin on the radioactive iodine sorption on activated carbon. J Radioanal Nucl Chem 328:659–667
Muhire C, Tesfay Reda A, Zhang D, Xu X, Cui C (2022) An overview on metal Oxide-based materials for iodine capture and storage. Chem Eng J 431:133816
Li D, Kaplan DI, Sams A, Powell BA, Knox AS (2018) Removal capacity and chemical speciation of groundwater iodide (I-) and iodate (IO3-) sequestered by organoclays and granular activated carbon. J Environ Radioact 192:505–512
Ikari M, Matsui Y, Suzuki Y, Matsushita T, Shirasaki N (2015) Removal of iodide from water by chlorination and subsequent adsorption on powdered activated carbon. Water Res 68:227–237
Li D, Kaplan DI, Price KA, Seaman JC, Roberts K, Xu C, Lin P, Xing W, Schwehr K, Santschi PH (2019) Iodine immobilization by silver-impregnated granular activated carbon in cementitious systems. J Environ Radioact 208–209:106017
Inglezakis VJ, Satayeva A, Yagofarova A, Tauanov Z, Meiramkulova K, Farrando-Perez J, Bear JC (2020) Surface Interactions and Mechanisms Study on the Removal of Iodide from Water by Use of Natural Zeolite-Based Silver Nanocomposites. Nanomaterials (Basel) 10:1156
Pham TCT, Docao S, Hwang IC, Song MK, Choi DY, Moon D, Oleynikov P, Yoon KB (2016) Capture of iodine and organic iodides using silica zeolites and the semiconductor behaviour of iodine in a silica zeolite. Energ Environ Sci 9:1050–1062
Chen J, Wang J, Gao Q, Zhang X, Liu Y, Wang P, Jiao Y, Zhang Z, Yang Y (2020) Enhanced removal of I- on hierarchically structured layered double hydroxides by in suit growth of Cu/Cu2O. J Environ Sci (China) 88:338–348
Kang J, Cintron-Colon F, Kim H, Kim J, Varga T, Du Y, Qafoku O, Um W, Levitskaia TG (2022) Removal of iodine (I- and IO3-) from aqueous solutions using CoAl and NiAl layered double hydroxides. Chem Eng J 430:132788
Chen J, Jiao Y, Chen K, Wang P, Wang J, Mao P, Jiang J, He M, Liu Y, Gong C, Yang Y (2021) Hierarchically mesoporous mixed copper oxide/calcined layered double hydroxides composites for iodide high-efficiency elimination. J Solid State Chem 303:122509
Lin Y, Jiang X, Kim ST, Alahakoon SB, Hou X, Zhang Z, Thompson CM, Smaldone RA, Ke C (2017) An elastic hydrogen-bonded cross-linked organic framework for effective iodine capture in water. J Am Chem Soc 139:7172–7175
Liu X, Zhang A, Ma R, Wu B, Wen T, Ai Y, Sun M, Jin J, Wang S, Wang X (2022) Experimental and theoretical insights into copper phthalocyanine-based covalent organic frameworks for highly efficient radioactive iodine capture. Chinese Chem Lett 33:3549–3555
Liu Q, Ma J, Dong Y (2011) Highly efficient iodine species enriching and guest-driven tunable luminescent properties based on a cadmium(II)-triazole MOF. Chem Commun (Camb) 47:7185–7187
Xu W, Zhang W, Kang J, Li B (2019) Facile synthesis of mesoporous Fe-based MOFs loading bismuth with high speed adsorption of iodide from solution. J Solid State Chem 269:558–565
Liu X, Verma G, Chen Z, Hu B, Huang Q, Yang H, Ma S, Wang X (2022) Metal-organic framework nanocrystals derived hollow porous materials: synthetic strategies and emerging applications. The Innovation :100281
Yang X, Liu X, Liu Y, Wang X, Chen Z, Wang X (2022) Optimizing iodine capture performance by metal-organic framework containing with bipyridine units. Front Chem Sci Eng :1–9
Zhang T, Yue X, Gao L, Qiu F, Xu J, Rong J, Pan J (2017) Hierarchically porous bismuth oxide/layered double hydroxide composites: Preparation, characterization and iodine adsorption. Clean Prod 144:220–227
Tian Z, Chee TS, Zhang X, Lei L, Xiao C (2021) Novel bismuth-based electrospinning materials for highly efficient capture of radioiodine. Chem Eng J 412:220–227
Liu L, Liu W, Zhao X, Chen D, Cai R, Yang W, Komarneni S, Yang D (2014) Selective capture of iodide from solutions by microrosette-like delta-Bi2O3. ACS Appl Mater Inter 6:16082–16090
Asmussen RM, Neeway JJ, Lawter AR, Wilson A, Qafoku NP (2016) Silver-based getters for 129I removal from low-activity waste. Radiochim Acta 104:905–913
Zhang Y, Liu H, Gao F, Tan X, Cai Y, Hu B, Huang Q, Fang M, Wang X (2022) Application of MOFs and COFs for photocatalysis in CO2 reduction, H2 generation, and environmental treatment. Energy Chem :100078
Cordova EA, Garayburu-Caruso V, Pearce CI, Cantrell KJ, Morad JW, Gillispie EC, Riley BJ, Colon FC, Levitskaia TG, Saslow SA, Qafoku O, Resch CT, Rigali MJ, Szecsody JE, Heald SM, Balasubramanian M, Meyers P, Freedman VL (2020) Hybrid Sorbents for 129I Capture from Contaminated Groundwater. ACS Appl Mater Inter 12:26113–26126
Levitskaia TG, Campbell EL, Hall GB, Chatterjee S, Boglaienko D, Reilly DD, Carlson MA (2020) Characterization of spent Purolite A530E resin with implications for long-term radioactive contaminant removal. J Environ Chem Eng 8:104155
Curtius H, Kattilparampil Z (2005) Sorption of iodine on Mg-Al-layered double hydroxide. Clay Miner 40:455–461
Zhao Q, Chen G, Wang Z, Jiang M, Lin J, Zhang L, Zhu L, Duan T (2021) Efficient removal and immobilization of radioactive iodide and iodate from aqueous solutions by bismuth-based composite beads. Chem Eng J 426:131629
Chen D, Yu C, Chang C, Wan Y, Frechet JM, Goddard WA 3rd, Diallo MS (2012) Branched polymeric media: perchlorate-selective resins from hyperbranched polyethyleneimine. Environ Sci Technol 46:10718–10726
Wei Y, Liu C, Mo L (2005) Ultraviolet absorption spectra of iodine, iodide ion and triiodide ion. Spectroscopy Spect Anal 25:86–88
Schmitz G (1999) Kinetics and mechanism of the iodate-iodide reaction and other related reactions. Phys Chem Chem Phys 1:1909–1914
Li J, Zhu L, Xiao C, Chen L, Chai Z, Wang S (2018) Efficient uptake of perrhenate/pertechnenate from aqueous solutions by the bifunctional anion-exchange resin. Radiochim Acta 106:581–591
Zhu Y, Gao N, Wang Q, Wei X (2015) Adsorption of perchlorate from aqueous solutions by anion exchange resins: Effects of resin properties and solution chemistry. Colloid Surf A 468:114–121
Chen J, Wang J, Gao Q, Zhang X, Liu Y, Wang P, Jiao Y, Zhang Z, Yang Y (2020) Enhanced removal of I- on hierarchically structured layered double hydroxides by in suit growth of Cu/Cu2O. J Environ Sci 88:338–348
Zhao X, Han X, Li Z, Huang H, Liu D, Zhong C (2015) Enhanced removal of iodide from water induced by a metal-incorporated porous metal-organic framework. Appl Surf Sci 351:760–764
Zhao Q, Chen G, Wang Z, Jiang M, Lin J, Zhang L, Zhu L, Duan T (2021) Efficient removal and immobilization of radioactive iodide and iodate from aqueous solutions by bismuth-based composite beads. Chem Eng Jl 426:131629
Tan K, Hameed BH (2017) Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J Taiwan Inst Chem Eng 74:25–48
Ho YS (2006) Review of second-order models for adsorption systems. J Hazard Mater 136:681–689
Al-Ghouti MA, Da’ana DA (2020) Guidelines for the use and interpretation of adsorption isotherm models: A review. J Hazard Mater 393:122383
Rajahmundry GK, Garlapati C, Kumar PS, Alwi RS, Vo DN (2021) Statistical analysis of adsorption isotherm models and its appropriate selection. Chemosphere 276:130176
Gu B, Brown GM, Bonnesen PV, Moyer LLY, BA, Ober R, Alexandratos SD, (2000) Development of novel bifunctional anion exchange resins with improved selectivity for pertechnetate sorption from contaminated groundwater. Environ Sci Technol 34:1075–1080
Li Y, Yang J, Jin J, Sun X, Wang L, Chen J (2014) New reversed-phase/anion-exchange/hydrophilic interaction mixed-mode stationary phase based on dendritic polymer-modified porous silica. J Chromatogr A 1337:133–139
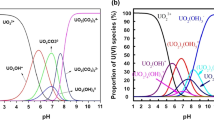
Baer MD, Pham V-T, Fulton JL, Schenter GK, Balasubramanian M, Mundy CJ (2011) Is iodate a strongly hydrated cation? J Phys Chem Lett 2:2650–2654
Marcus Y (1997) Ion properties. CRC Press
Chen JY, Gu AT, Miensah ED, Liu Y, Wang P, Mao P, Gong CH, Jiao Y, Chen K, Zhang ZX, Yang Y (2021) Core-shell ZnO@Cu2O encapsulated Ag NPs nanocomposites for photooxidation-adsorption of iodide anions under visible light. Sep Purifi Technol 262:118328
Li J, Dai X, Zhu L, Xu C, Zhang D, Silver MA, Li P, Chen L, Li Y, Zuo D, Zhang H, Xiao C, Chen J, Diwu J, Farha OK, Albrecht-Schmitt TE, Chai Z, Wang S (2018) 99TcO4- remediation by a cationic polymeric network. Nat Commun 9:3007
Zhang Y, Cremer PS (2010) Chemistry of Hofmeister anions and osmolytes. Annu Rev Phys Chem 61:63–83
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22176139, 21790374, 21825601, and U1967217), the Postdoctoral Science Foundation of China (2021M702390 and BX2021206), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the National Key R&D Program of China (2021YFB3200400 and 2018YFB1900203).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuting Zhao, Jie Li, and Long Chen contributed equally to this paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Li, J., Chen, L. et al. Efficient removal of iodide/iodate from aqueous solutions by Purolite A530E resin. J Radioanal Nucl Chem 332, 1193–1202 (2023). https://doi.org/10.1007/s10967-023-08786-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08786-8