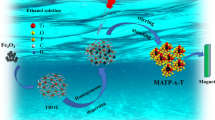
Abstract
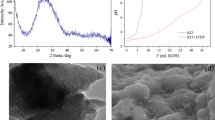
Amorphous titanium phosphates (ATP) doped with NH4+, K+, Na+, and Li+ ions (ATP-M, M = NH4, K, Na, and Li) were prepared by a co-precipitation method. The SEM&EDS, TGA, XRD characterizations displayed that ATP-M was successfully synthesized, and the doping of NH4+, K+, Na+, and Li+ ions did not change the crystal form of ATP. The results revealed that the optimum pH value for U(VI) extraction on ATP-M was determined to be 5.0. Moreover, the saturated adsorption capacity obtained by the Langmuir equation for ATP-NH4, ATP-K, ATP-Na, and ATP-Li was 505.1, 366.1, 273.3, and 144.1 mg g−1, respectively. Furthermore, the interaction between ATP-M and U(VI) was discussed. In short, ATP-NH4 may be an effective adsorbent to remove of U(VI) in wastewater due to its high adsorption ability.







Similar content being viewed by others
References
Cui WR, Li FF, Xu RH, Zhang CR, Chen XR, Yan RH, Liang RP, Qiu JD (2020) Regenerable covalent organic frameworks for photo-enhanced uranium adsorption from seawater. Angew Chem Int Ed 132(40):17837–17843. https://doi.org/10.1002/anie.202007895
Gupta DK, Vuković A, Semenishchev VS, Inouhe M, Walther C (2020) Uranium accumulation and its phytotoxicity symptoms in Pisum sativum L. Environ Sci Pollut Res 27(3):3513–3522. https://doi.org/10.1007/s11356-019-07068-9
Sahu MS, Sar SK, Dewangan R, Baghel T (2020) Health risk evaluation of uranium in groundwater of Bemetara district of Chhattisgarh state, India. Environ Dev Sustain 22(8):7619–7638. https://doi.org/10.1007/s10668-019-00539-6
Rani R, Munjal N, Kamboj U (2021) A review on different techniques for analysis for uranium as a ground water contaminant. Adv Funct Mater Devices 14:197–204. https://doi.org/10.1007/978-981-16-5971-3_22
Prasad M, Kumar GA, Sahoo SK, Ramola RC (2019) Health risks associated with the exposure to uranium and heavy metals through potable groundwater in Uttarakhand state of India. J Radioanal Nucl Chem 319(1):13–21. https://doi.org/10.1007/s10967-018-6281-7
Wang T, Wu D, Wang YL, Huang TB, Histand G, Wang TT, Zeng HP (2018) One-step solvothermal fabrication of Cu@PANI cor-shell nanospheres for hydrogen evolution. Nanoscale 10(46):22055–22064. https://doi.org/10.1039/c8nr06245e
Janusz W, Khalameida S, Skwarek E, Sydorchuk V, Charmas B (2019) The effect of hydrothermal modification of titanium phosphate on the adsorption affinity towards cadmium ions. Physicochem Probl Min Process 55(6):1568–1576. https://doi.org/10.5277/ppmp19088
Maheria K, Chudasama U (2006) Studies on kinetics, thermodynamics and sorption characteristics of an inorganic ion exchanger-Titanium phosphate towards Pb (II), Bi (III) and Th (IV). J Indian Inst Sci 86(5):515–525
Jia K, Pan BC, Zhang WM, Jiang PJ, Hong CH, Pan BJ, Zhang QX (2008) Adsorption of Pb2+, Zn2+, and Cd2+ from waters by amorphous titanium phosphate. J Colloid Interface Sci 318(2):160–166. https://doi.org/10.1016/j.jcis.2007.10.043
Tang ML, Chen J, Wang PF, Wang C, Ao YH (2018) Highly efficient adsorption of uranium(VI) from aqueous solution by a novel adsorbent: titanium phosphate nanotubes. Environ Sci Nano 5(10):2304–2314. https://doi.org/10.1039/C8EN00761F
Wang YQ, Xie YH, Zheng ZY, Zeng DJ, Dai Y, Zhang ZB, Cao XH, Zou R, Liu YH (2021) Surfactant-assisted adsorption of uranyl ions in aqueous solution on TiO2/polythiophene nanocomposite. Environ Sci Pollut Res 28(28):37182–37194. https://doi.org/10.1007/s11356-021-12587-5
Wang R, Ye JW, Rauf A, Wu X, Liu HX, Ning G, Jiang H (2016) Microwave-induced synthesis of pyrophosphate Zr1-xTixP2O7 and TiP2O7 with enhanced sorption capacity for uranium (VI). J Hazard Mater 315:76–85. https://doi.org/10.1016/j.jhazmat.2016.03.092
Guo SY, Han S (2014) Constructing a novel hierarchical 3D flower-like nano/micro titanium phosphate with efficient hydrogen evolution from water splitting[J]. J Power Sources 267:9–13. https://doi.org/10.1016/j.jpowsour.2014.05.011
Maslova MV, Rusanova D, Naydenov V, Antzutkin ON, Gerasimova LG (2008) Synthesis, characterization, and sorption properties of amorphous titanium phosphate and silica-modified titanium phosphates. Inorg Chem 47(23):11351–11360. https://doi.org/10.1021/ic801274z
Maslova MV, Chugunov AS, Gerasimova LG, Konovalova NV (2013) Acid-base and sorption properties of amorphous titanium phosphate. Radiochemistry 55:392–398. https://doi.org/10.1134/S1066362213040097
Zakutevskyy OI, Psareva TS, Strelko VV (2012) Sorption of U(VI) ions on sol-gel-synthesized amorphous spherically granulated titanium phosphates. Russ J Appl Chem 85:1366–1370. https://doi.org/10.1134/S107042721209011X
Janusz W, Khalameida S, Skwarek E, Skubiszewska-Zięba J, Sydorchuk V, Charmas B (2019) Modification of titanium phosphate precipitated from titanylsulfate. J Therm Anal Calorim 135(6):2925–2934. https://doi.org/10.1007/s10973-018-7611-2
Garcia-Glez J, Amghouz Z, Khainakov SA, Espina A, Alfonso BF, Trobajo C (2014) Ammonium-exchanged phase of gamma-titanium phosphate Hydrothermal synthesis, crystal structure, and thermal behavior. J Therm Anal Calorim 118(2):783–791. https://doi.org/10.1007/s10973-014-3923-z
Guo SY, Han S, Chi B, Pu J, Li J (2014) Synthesis of shape-controlled mesoporous titanium phosphate nanocrystals: the hexagonal titanium phosphate with enhanced hydrogen generation from water splitting. Int J Hydrog Energy 39(6):2446–2453. https://doi.org/10.1016/j.ijhydene.2013.12.007
Zhuravlev I, Zakutevsky O, Psareva T, Kanibolotsky V, Strelko V, Taffet M, Gallios G (2002) Uranium sorption on amorphous titanium and zirconium phosphates modified by Al3+ or Fe3+ ions. J Radioanal Nucl Chem 254(1):85–89. https://doi.org/10.1023/A:1020893515049
Bortun AI, Bortun LN, Clearfield A, Khainakov SA, Strelko VV, Khryashevskii VN, Kvashenko AP, Voitko II (1997) Synthesis and characterization of ion exchange properties of spherically granulated titanium phosphate. Solvent Extr Ion Exch 15(3):515–532. https://doi.org/10.1080/07366299708934491
Maslova MV, Rusanova D, Naydenov V, Antzutkin ON, Gerasimova LG (2012) Extended study on the synthesis of amorphous titanium phosphates with tailored sorption properties. J Non Cryst Solids 358(22):2943–2950. https://doi.org/10.1016/j.jnoncrysol.2012.06.033
Sabur AM, Rdaiaan MA (1879) Design, synthesis and biological screening of new benzimidazole derivatives. J Phys Conf Ser 2:022056. https://doi.org/10.1088/1742-6596/1879/2/022056
Wang QF, Ling Z, Sun JQ, Shen JC (2005) A facile layer-by-layer adsorption and reaction method to the preparation of titanium phosphate ultrathin films. Chem Mater 17(13):3563–3569. https://doi.org/10.1021/cm050646w
Ortíz-Oliveros HB, Flores-Espinosa R, Ordoñez-regil E, Fernández-Valverde S (2014) Synthesis of α-Ti(HPO4)2·H2O and sorption of Eu (III). Chem Eng J 236:398–405. https://doi.org/10.1016/j.cej.2013.09.103
Sahu BB, Parida K (2002) Cation exchange and sorption properties of crystalline alpha-titanium(IV) phosphate. J Colloid Interface Sci 248(2):221–230. https://doi.org/10.1006/jcis.2001.7818
Pironon J, Pelletier M, Donato PD, Mosser-Ruck R (2003) Characterization of smectite and illite by FTIR spectroscopy of interlayer NH4+ cations. Clay Miner 38(2):201–211. https://doi.org/10.1180/0009855033820089
Janiszewska E, Kot M, Zieliński M (2018) Modification of silica with NH4+ agents to prepare an acidic support for iridium hydrogenation catalyst. Microporous Mesoporous Mater 255:94–102. https://doi.org/10.1016/j.micromeso.2017.07.031
Yuan JY, Yang J, Ma HW, Liu CJ (2016) Crystal structural transformation and kinetics of NH4+/Na+ ion-exchange in analcime. Microporous Mesoporous Mater 222:202–208. https://doi.org/10.1016/j.micromeso.2015.10.020
Jiang D, Chen M, Wang H, Zeng GM, Huang DL, Cheng M, Liu Y, Xue WJ, Wang ZW (2019) The application of different typological and structural MOFs-based materials for the dyes adsorption. Coord Chem Rev 380:471–483. https://doi.org/10.1016/j.ccr.2018.11.002
Jun BM, Lee HK, Park SB, Kim TJ (2021) Purification of uranium-contaminated radioactive water by adsorption: a review on adsorbent materials. Sep Purif Technol 278:119675. https://doi.org/10.1016/j.seppur.2021.119675
Bortun A, Strelko VV, Jaimez E, Garcia JR, Rodriguez J (1995) Synthesis of semicrystalline materials by organic compound intercalation into amorphous titanium phosphate. Chem Mater 7(2):249–251. https://doi.org/10.1021/cm00050a002
Xiao J, Jing Y, Yao Y, Wang XQ, Jia YZ (2019) Synthesis of amidoxime-decorated 3D cubic mesoporous silica via self-assembly co-condensation as a superior uranium(VI) adsorbent. J Mol Liq 277:843–855. https://doi.org/10.1016/j.molliq.2019.01.009
Budnyaka TM, Strizhak AV, Agnieszka GP, Sternikc D, Komarov IV, Kołodynska D, Majdanc M, Tertykha VA (2016) Silica with immobilized phosphinic acid-derivative for uranium Extraction. J Hazard Mater 314:326–340. https://doi.org/10.1016/j.jhazmat.2016.04.056
Wang YQ, Zeng DJ, Dai Y, Fang CL, Han XW, Zhang ZB, Cao XH, Liu YH (2020) The adsorptive ability of 3D flower-like titanium phosphate for U (VI) in aqueous solution. Water Air Soil Pollut 231(9):1–12. https://doi.org/10.1007/s11270-020-04817-2
Xie JH, Dai Y, Wang YQ, Zhang ZB, Wang YC, Tao QQ, Liu YH (2021) Facile immobilization of NiFeAl-LDHs into electrospun poly (vinyl alcohol)/poly (acrylic acid) nanofibers for uranium adsorption. J Radioanal Nucl Chem 329(2):1103–1117. https://doi.org/10.1007/s10967-021-07860-3
Singh AK, Gonuguntla S, Mahajan B, Pal U (2019) Noble metal-free integrated UiO-66-PANI-Co3O4 catalyst for visible-light-induced H2 production. Chem Commun 55(96):14494–14497. https://doi.org/10.1039/c9cc07414g
Khraisheh M, Al-Degs Y, Allen S, Ahmad M (2002) Elucidation of controlling steps of reactive dye adsorption on activated carbon. Ind Eng Chem Res 41(6):1651–1657. https://doi.org/10.1021/ie000942c
Wang N, Chen J, Wang JN, Feng JT, Yan W (2019) Removal of methylene blue by Polyaniline/TiO2 hydrate: adsorption kinetic, isotherm and mechanism studies. Powder Technol 347:93–102. https://doi.org/10.1016/j.powtec.2019.02.049
Jasni MJF, Arulkumar M, Sathishkumar P, Yusoff ARM, Buang NA, Gu FL (2017) Electrospun nylon 6,6 membrane as a reusable nano-adsorbent for bisphenol A removal: adsorption performance and mechanism. J Colloid Interface Sci 508:591–602. https://doi.org/10.1016/j.jcis.2017.08.075
Oguz E (2005) Adsorption characteristics and the kinetics of the Cr(VI) on the Thuja oriantalis. Colloids Surf A 252(2):121–128. https://doi.org/10.1016/j.colsurfa.2004.10.004
Rahman N, Haseen U (2014) Equilibrium modeling, kinetic, and thermodynamic studies on adsorption of Pb(II) by a hybrid inorganic-organic material: polyacrylamide zirconium(IV) iodate. Ind Eng Chem Res 53(19):8198–8207. https://doi.org/10.1021/ie500139k
Cheng L, Cui W, Cheng Z, Wang Y, Xu L, Zhang Z, Chen L, Luo Q, Cao X, Liu Y (2022) An effective magnetic amorphous titanium phosphate material to remove U(VI) from water: synthesis, characterization, and adsorption properties. J Radioanal Nucl Chem 331:4705–4719. https://doi.org/10.1007/s10967-022-08572-y
Jiang W, Saxena A, Song BK, Ward BB, Beveridge TJ, Myneni SCB (2004) Elucidation of functional groups on gram-positive and gram-negative bacterial surfaces using infrared spectroscopy. Langmuir 20(26):11433–11442. https://doi.org/10.1021/la049043+
Jiao Z, Meng YG, He CL, Yin XB, Wang XP, Wei YZ (2021) One-pot synthesis of silicon-based zirconium phosphate for the enhanced adsorption of Sr (II) from the contaminated wastewater. Microporous Mesoporous Mater 318:111016. https://doi.org/10.1016/j.micromeso.2021.111016
Miao ZC, Li ZB, Liang MF, Meng J, Zhao YZ, Xu LL, Mu JL, Zhuo SP, Si WJ (2020) Ordered mesoporous titanium phosphate material: a highly efficient, robust and reusable solid acid catalyst for acetalization of glycerol. Chem Eng J 381:122594. https://doi.org/10.1016/j.cej.2019.122594
Do J, Son N, Chava R, Mandari KK, Pandey S, Kumaravel V, Senthil TS, Joo S, Kang M (2020) Plasmon-induced hot electron amplification and effective charge separation by Au nanoparticles sandwiched between copper titanium phosphate nanosheets and improved carbon dioxide conversion to methane. Acs Sustain Chem Eng 8(50):18646–18660. https://doi.org/10.1021/acssuschemeng.0c06983
Cai BY, Jiang N, Tan PJ, Hou Y, Li YB, Zhang L, Zhu SS (2019) The custom making of hierarchical micro/nanoscaled titanium phosphate coatings and their formation mechanism analysis. RSC Adv 9(70):41311–41318. https://doi.org/10.1039/C9RA08168B
Cai YW, Wu CF, Liu ZY, Zhang LJ, Chen LH, Wang JQ, Wang XK, Yang ST, Wang SA (2017) Fabrication of a phosphorylated graphene oxide-chitosan composite for highly effective and selective capture of U(VI). Environ Sci Nano 4(9):1876–1886. https://doi.org/10.1039/C7EN00412E
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21866003, 21906017), Jiangxi Provincial Natural Science Foundation (Grant Nos. 20202BABL213026 and 20202BABL203016).
Author information
Authors and Affiliations
Contributions
LX wrote the entire draft of the manuscript. Written review and editing of the article by YW. The core conceptual idea and study design were all provided by XC and YL. The preparation and adsorptive experiments were conducted out with WC. Material preparation and data analysis and characterization analysis of the adsorbents was worked out through QL and LC.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no conflicts of interest to this work. The manuscript is approved by all authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiong, L., Wang, Y., Cui, W. et al. Preparation of ion-doped amorphous titanium phosphates and their adsorption properties for U(VI). J Radioanal Nucl Chem 332, 1303–1314 (2023). https://doi.org/10.1007/s10967-023-08778-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08778-8